Kite Pharma Part 2: An Overview of CAR-T Cell Drug Development Efforts
Overview
This section tries to give a brief, layman’s overview on the potential role of CAR-T cells in treating certain hematological cancers and the competitive dynamics for companies operating in this space. Kite’s first product is the CAR-T drug KTE-C19.
Key Points;
- There are three CAR-T drugs in development from Kite, Juno and Novartis. They have similar mechanisms of action in that they target the CD-19 antigen on cancer cells. Nothing in the limited clinical data created in the small phase 1/2 trials conducted so far indicates that these products are meaningfully differentiated.
- Each of these drugs have produced dramatic responses in r/r ALL and to a lesser extent in r/r DLBCL patients who no longer responded to existing drug regimens or who had relapsed after a stem cell transplant. They are viewed as a major advance by key opinion leaders. Their major drawback is that they cause a troubling incidence of life threatening side effects that limit their use.
- These drugs are only effective against certain types of hematological cancers that express CD-19 and that account for less than 4% of all cancers. Existing drug regimens are curative in a significant percentage of these cancer patients. The unmet medical need that CAR-T cells address is in relapsed or refractory patients.
- The treatment regimen starts with a highly toxic chemotherapy regimen to deplete the native T-cell population. This is then followed by a single infusion of CAR-T cells. Both of these treatments cause life threatening side effects. This will likely limit their use even in the relapsed/ refractory patients. In clinical trials so far, patients are hospitalized as a protocol of the trial in order to deal with side effects.
- The lay media has sometimes extrapolated the effectiveness of CAR-T cells in certain hematological cancers to having comparable effectiveness in all cancers. However, in solid tumors, the role of CAR-T treatment is likely to have a limited, if any role.
- All of the basic research on CAR-T that Novartis, Kite and Juno rely on to design their products originated at NCI and academic centers who have also conducted all of the initial clinical trials in humans. Neither Kite or Juno or Novartis have developed a product on their own.
- Kite, Juno and Novartis are each conducting phase 2 trials that they claim will be sufficient for registration. They are open label trials that have no control arm and their primary efficacy endpoints are measures of tumor shrinkage as opposed to hard endpoints like overall survival and progression free survival.
- Kite believes that it can file a BLA based on an interim look at the ZUMA-1 phase 2 for the treatment of r/r DLBCL. It then projects approval in mid- 2017. If the trial runs to its scheduled date for completion of March 2017, the potential approval would be stretched to late 2017 or early 2018.
- Juno had originally planned to submit a BLA for adult r/r ALL in late 2016 or early 2017 and gain approval in mid to late 2017. However, a clinical hold on the trial will now delay potential approval until mid-2018.
- Novartis will file for approval for pediatric r/r ALL in 2017 and probably may gain approval in 2017. They are less specific on this than Kite and Juno. Novartis is also conducting a phase 2 trial in r/r DLBCL that may be on the same timeline as ZUMA-1.
- The plan of all three companies is to ultimately gain approval in a broad number of hematological tumor indications.
- First mover advantage in the CAR-T space will be very important for commercial success. I think that Novartis is most likely to gain this status although Kite potentially could be the first mover if its interim analysis strategy works. My best guess is that Novartis gains approval for r/r ALL and r/r DLBCL at about the same time as Kite gains approval for r/r DLBCL. Juno looks like the third place finisher in a three horse race and this is a major commercial disadvantage.
CAR-T Cells- A Brief Overview
How CAR-T Cells Are Created
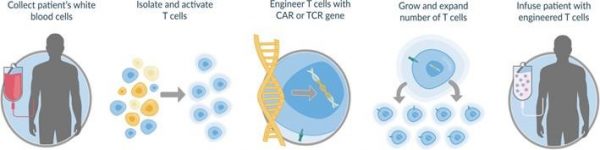
Over the last two years there has been great excitement in the medical and investment community about engineered autologous T-cells (eACTS), of which chimeric antigen receptor T-cells (CAR-T) are the first generation of products. This starts by taking living T-cells which are key elements of the body’s immune response from the body. They are then enhanced for both specificity and efficacy against certain cancer targets. This engineering involves a tour de force of technologies that biotechnology has created including recombinant DNA, monoclonal antibodies and gene transfer. The uniqueness of eACTS is that they combine the cancer killing power of T-cells with the specificity of antibodies.
After the cells are genetically engineered, they are induced to divide ex vivo (outside the body) into billions of clones (identical cells). The cells are then reinfused into the body where they continue to divide rapidly. Before CAR-T cell infusion, an intense chemotherapy regimen is used to deplete the natural T-cell population and in doing so to enhance the rate of division. This is shown below:
Clinical Data on CAR-T Cells Comes from NCI and Academic Centers. Not Kite, Juno and Novartis
The NCI has been conducting research on eACTS for over 20 years. The first humans were treated in 2010, but there was limited medical or investor interest until 2014, when results from two phase 1 studies using CAR-T cells were published. A trial from NCI was published in Lancet and another from a collaboration of Children’s Hospital of Philadelphia and the University of Pennsylvania (that was based on NCI technology) was published in the New England Journal of Medicine. Both studies were done in refractory/ relapsed acute lymphocytic leukemia patients who had exhausted all chemotherapy treatment options and in some cases had relapsed after a stem cell transplant.
The outlook for these patients was extremely grim with average life expectancies of perhaps a half year or less. For 20 patients treated in the NCI trial 14 (70%) had a complete response (CR). In the CHOP/U of P study of 30 patients, 27 (90%) had a CR. (A CR is defined as the cancer being undetectable via MRI and CT imaging although undetectable metastases can remain.) These results were considered to be remarkable by key opinion leaders as achieving a CR can significantly extend life for the patient and in some cases can signal a cure.
On the investment front, venture capitalists formed two biotechnology companies, Kite and Juno, around this technology. Taking advantage of investor excitement, they executed initial public offerings in 2014. The third entrant in CAR-T development, Novartis, licensed technology from the Children’s Hospital of Philadelphia/ University of Pennsylvania collaboration. Kite was founded in 2009, but in early 2014 there were only 10 employees and the CEO and CSO just joined the Company in 2014.Venture capitalists formed Juno in 2013 just one year before its IPO. Kite had gained access to NCI’s research through a CRADA signed in 2012 and Juno obtained its technology through licensing agreements with several academic centers in 2013.
Investors were so mesmerized by the phase 1 results in r/r ALL the NCI and CHOP/ U of P trials that Juno and Kite were able to come public at lofty valuations Interestingly, at the time of their offerings, neither company had ever started, let alone completed a human clinical trial and even more interestingly, neither company has subsequently completed a human clinical trial although each has phase 2 trials in progress. Since their IPOS, both companies have had easy access to capital that has led to large secondary offerings.
My View on the Potential for CAR-T
I am much less excited by the CAR-T technology than the market as a whole which has resulted in lofty valuations for Kite and Juno. First of all, I must emphasize that I view the phase 1 results and additional investigator results of CAR-T drugs as being very impressive and believe that they represent a major breakthrough in treating r/r ALL. More importantly, key opinion leaders view the results in the same way.
However, my view is that the stocks have been hyped by managements, venture capitalists and investment firms raising capital for them so that investors may not understand that this is a technology that is still in a very early stage of development. It will take decades to refine this technology and in this very early stage, there are many unresolved issues. In biotechnology there are always bumps and sometimes steep mountains along the development road. I think that eACTs will face some mountain ranges before their real potential is realized. While Kite and Juno are current market leaders and are very well capitalized, based on biotechnology history this does not mean they will be the ultimate winners.
If you want additional information on engineered autologous T-cells (eACTS), I suggest that you read my report of July13, 2016, “A Non-Consensus, More Balanced Look at the CAR-T Development Efforts of Kite and Juno” before proceeding. That report was written for laymen by a lay person (me).
Brief Comments on the Role of CAR-T Treatment in Cancer
I apologize to anyone who thinks I am speaking down to them, but I must first emphasize the heterogeneity of cancers. The reason for doing this is that some investors seem to believe that the promising efficacy results seen with CAR-T cells in r/r ALL is a proxy for what will be seen in all cancers. This is very much not the case. The first generation CAR-T products may only be applicable in less than 1% of cancers. Significant technological advances have to be made for CAR-T in particular and eACT technology to be broadly used in treating solid tumors.
Let me digress for a moment to talk about an issue that always rankles me. I hear fund raising organizations like the American Cancer Society ask for funds to find a cure for cancer. They try to give the grossly misleading impression that cancer is a disease like hypertension, which may result from several causes, but its symptoms can generally be treated with a limited number of drugs. The American Cancer website raised $858 million in 2015. It then spent $144 million (17% of its funds) on basic research which compares to $226 million (26%) on salaries and fund raising. The claim that contributing to the American Cancer Society will lead to a cure for cancer is patently false. It costs perhaps $500 million to $1 billion to develop a drug for one of literally hundreds of cancers. Giving money to the American Cancer Society is almost certainly not going to lead to finding a cure for cancer. You might want to contribute directly to the National Cancer Institute where all of you money goes to basic research, but that is another story.
I went on this tirade because it may be illustrative of a wrong view by society about cancer. It is different from hypertension. Cancer basically results from mutations in normal cells that cause them to grow uncontrollably. Cells in the body are quite diverse as are the mutations that may occur that can lead to what we refer to as cancer. Cancers are broadly classified into hematological tumors that affect the white blood cells that are formed in the bone marrow and circulate in the blood and lymph and solid tumors such as breast or prostate cancer. Because cell types are so different and the mutations that cause cancer in them are so different, the many, many cancers require many, many different treatments. Also, treatments for individual cancers usually require combinations of drugs that can vary widely from one cancer type to the next. It goes unsaid that a curative treatment in one type of cancer may be totally ineffective in another.
It is also the case that the use of cancer drugs is limited by their toxicities. They are, after all, poisons that are designed to kill cancer cells. However, these drugs are usually not specific to cancer cells and the mechanism that is used to attack cancer cells can and does kill normal cells. Hence, side effects caused by a drug are a limiting factor. The physician has to weigh off the damage caused to normal cells versus the killing of cancer cells. The CAR-T products have life threatening side effects that has to be figured in the decision to use them.
The Cancer Target of the Three CAR-T Cell Products in Clinical Development is CD-19
CAR-T cells drugs now in development from Kite, Novartis and June all have the same mechanism of action. It is unclear at this point as to whether they have any meaningful differentiations. They each target and destroy cells that express CD-19 on their surface. This means that they can address certain hematological (blood) tumors that are responsible for less than 4% of all cancers. These three products are not effective against cancers which do not express CD-19 which is the case with all solid tumors. Other types of CAR-T products may or may not have efficacy in a very limited number of solid tumors.
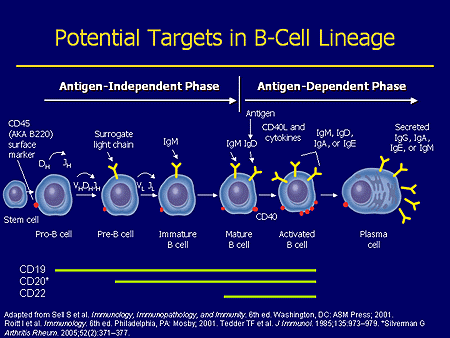
The three CAR-T products are genetically engineered to have a receptor on their surface that seeks out the CD-19 antigen. CD-19 is a molecular complex that occurs on the cell surface of the various cells in the B-cell lineage with the exception of: (1) the stem cell that gives rise to them and (2) the plasma cell which is the last cell in the lineage that produces antibodies (each plasma cell produces one specific antibody). This is shown in the following image. You can also see that the CD-19 marker does not occur in the stem cell nor in the plasma cell, but it does occur in all of the cell lineages in between. As shown in the following image, these are the pro-B-cell, pre-B-cell, immature B-cell, mature-B-cell and activated B-cell. It is in these cells that various lymphomas and leukemias occur.
The CAR-T cell receptor binds to CD-19 and among other things releases cytokines that destroy the cell on which the CD-19 antigen occurs. It is important to understand that the CD19 molecular complex occurs not only on cancerous cells, but also normal cells. The CAR-T drug essentially destroys most of the normal as well as the cancerous cells.
Because B-cells are responsible for creating antibodies against infectious diseases, the patient is often given immunoglobulins as prophylaxis against infections. This seems at first thought to be pretty dangerous to shut down the body’s ability to create antibodies. However, this mechanism of action is well tested as this is what the very broadly used cancer drug Rituxan does. Rituxan attaches to the CD 20 complex of B-cells and like CAR-T cells destroys both normal and cancerous cells. Over two decades of use, Rituxan has been proven to be safe and effective in treating B-cell cancers. The difference between Car-T cells and Rituxan is that the CAR-T cells are more potent. You can think of Rituxan as a 500 pound bomb and the CAR-T as a 5,000 pound blockbuster.
Side Effects of CAR-T Cells
With the greater potency of CAR-T cells comes a price to be paid in terms of side effects. The phase 1 trials showed that the CAR-T cells can lead to cytokine release syndrome and this resulted in life threatening side effects in both trials as well as a broad range of other side effects. Investigators were generally able to manage these side effects and they were transient. Both Juno and Kite maintain that techniques have been developed to reduce the intensity of the cytokine release syndrome, but this has not yet been vetted and shown in a clinical trial setting.
Importantly, cell infusion is only the one of a two part treatment regimen. Treatment starts with an aggressive chemotherapy pre-conditioning program designed to deplete T-cells in the body prior to infusion of CAR-T cells. Patients are then hospitalized and given a CAR-T cell infusion. My initial impression was that the whole treatment regimen consisted of a single infusion of CAR-T cells.
What Medical Need Do the CAR-T cells Meet?
Two of the important cancers in which CAR-T cells have been tested are pediatric acute lymphocytic leukemia and diffuse large B-cell lymphoma. In pediatric ALL the cure rate with the current standard of care chemotherapy regimen is about 80%. In DLBCL, the current cure rate with the standard of care regiment is about 60%. This means that CAR-T cells at least initially will only be used in patients who have failed standard of care treatment.
The commonly accepted approach to developing a new drug is to use it in situations in which there is no effective therapy. In the case of both pediatric ALL and DLBCL this was in relapsed/ refractory patients who had failed standard of care and in some cases stem cell transplants; these patients had no viable therapeutic option. As I previously mentioned, the efficacy was extremely positive in this group and clearly CAR-T cells meets an unmet medical need in this patient population.
The key question that is as yet unanswered is whether CAR-Ts have a wider role to play in less severe cases of pediatric ALL and DLBCL. This will likely depend on how physicians view the balance of efficacy against life with accompanying life threatening side effects of CAR-T cells versus the very effective chemotherapy regimens now used. Over time, it may be the case that the side effect issues with CAR-Ts can be managed and allow wider use, but there is also a case to be made that their toxicities will limit their use to relapsed/ refractory disease.
CAR-T Cells Have a Limited or Perhaps no Role in Solid Tumors
CAR-T cells targeting CD-19 are perfect for many B-cell cancers. However, this appears to be a very unique situation. As I previously mentioned CAR-T cells seek out and destroy normal cells expressing CD-19 as well as cancerous cells. This destruction of normal cells is an acceptable risk in B-cell cancers (recall the previously discussed Rituxan example). However, it is probable that this is a unique situation.
Let me give you an example. Novartis designed a CAR-T specific to HER-2, an antigen that is widely expressed in some breast cancers. It was quite effective, but HER-2 is also expressed in normal tissues in the lung. The side effects caused by damage to the lung was not acceptable. In my layman’s opinion, it is not likely that CAR-T cells will be found to be a good therapeutic option in solid tumors. However, there is clinical work going on in some select situations in which an antigen is highly expressed in cancer cells, but not normal cells.
Current Leaders in CAR-T Technology?
The three front runners in developing CAR-T therapies are Novartis, Kite and Juno. These companies gained access to their technologies from NCI in the case of Kite and academic centers in the case of Novartis and Juno. None of these companies has completed a trial using CAR-T cells. The phase 1 trials and individual case studies were all done at NCI or academic centers. These three companies built on that work to launch phase 2 trials that they think could be pivotal.
All of the phase 2 trials are open label trials and are using objective response rate (ORR) as the primary endpoint. Novartis first phase 2 trials are in pediatric r/r ALL and r/r DLBLC; it plans to file for regulatory approval in 2017. Kite is also running a trial in DLBCL and says that it expects that the results from an interim efficacy look that should shortly take place will allow them to file a BLA in late 2016 and gain approval in mid-2017. Otherwise, the trial is scheduled to end in March 2017 and this would result in potential approval in late 2017 or early 2018. Juno is running a trial in adult r/r ALL and originally predicted approval in 2017. However, the recent clinical hold will push potential approval to mid-2018.
There could be important differences in the three CAR-T products. Remember that the different manufacturing processes used to make CAR-T products could result in products with different characteristics, but there is no way of predicting this. Because results seen in the phase 1 and case studies were pretty similar, it is probably the case that the three products in phase 2 trials will also be similar.
The Technology Base: National Cancer Institute (NCI) is the Research Engine for KITE
Kite’s technology is based on pioneering work on engineered autologous T-cells done over the last 20 years at the National Cancer Institute in the laboratory of Dr. Steven Rosenberg. Kite signed a five year cooperative research and development agreement (CRADA) with the NCI in September 2012. In this agreement, Kite and the NCI agreed to work together to: (1) develop new CAR and TCR constructs, (2) develop and optimize the manufacturing process and (3) identify new antigen targets.
Kite provides research funding for a research plan that is jointly developed by Kite and NCI in which NCI manages research activities from pre-clinical discovery through phase 1 trials. Because NCI has a close relationship (based on its great credibility) with FDA, Kite believes NCI can make expeditious adjustments in early stage clinical trials as they progress which a private company could not, thus saving significant amounts of time. Kite has stated that it believes that a product can be moved from NCI conducted phase 1 trials to a KITE conducted phase 2 trial based on results in as few as 5 to 7 patients.
Patents stemming from the CRADA can be owned by the NCI, Kite or jointly depending on how the invention originated. NCI notifies Kite of all of its patent applications and Kite is entitled to the right of first negotiation on a license to that patent. Kite is given access to all the data supporting the patent and has 4 months from patent filing to inform NCI that it wants to license the patent(s). It then has 10 months to negotiate a license.
Kite is also entitled to ownership of all data resulting from the CRADA. This is important if Kite decides not to license the technology or cannot come to terms with the NCI. It means that competitors will not have access to this data in considering a license and may have to replicate all CRADA funded preclinical and Phase 1 trials.
Tagged as CAR-T cells, KITE, Kite Pharma, KTE-C19 + Categorized as Company Reports, LinkedIn