Cytokinetics: Based on Data from Fortitude ALS Trial, Cytokinetics and Astellas Indicate They Will Undertake Phase 3 Trial of Reldesemtiv in ALS (CYTK, Buy, $9.30)
Key Points:
- Trial missed achieving statistical significance on primary endpoint of slow vital capacity
- Still, key opinion leaders when looking at the totality of data are strongly supportive of launching a phase 3 trial
- Management has not yet issued guidance, but my guess is that the trial could begin in late 2019 and conclude in 2H, 2021, about the same time we will see topline data from omecamtiv mecarbil in the phase 3 GALACTIC-HF trial
- ALS has been an incredibly difficult disease for drug development and there remains meaningful risk of failure, but there is also meaningful hope for success
- Success in the upcoming phase 3 trial could lead to $1 billion plus US sales opportunity for reldesemtiv
- My buy recommendation is more than supported by the prospects for omecamtiv with reldesemtiv providing yet more potential.
At First Glance, Press Release Seemed to Indicate Trial Results were Disappointing
I was coming back from a West Coast trip last Sunday night and on the ride home from the airport, I was catching up on my e-mails. I saw that Cytokinetics had issued a press release “Cytokenetics Announces Results of FORTITUDE-ALS, a Phase 2 Clinical Trial of Reldesemtiv in Patients with ALS, Presented at American Academy of Neurology Annual Meeting”. The bullet points were:
- Trial Did Not Meet Statistical Significance for Primary Efficacy Analysis,
- Patients on All Doses of Reldesemtiv Declined Less Than Patients on Placebo for SVC and ALSFRS-R, With Clinically Meaningful Differences Emerging Over Time,
- Early Terminations and Serious Adverse Events Were Balanced Across Treatment Arms
At first glance, my take was that CYTK was indicating that the trial results were disappointing. This would not be surprising considering the failure of tirasemtiv in the phase 3 VITALITY-ALS trial that reported out in November 2017. Both drugs have the same mechanism of action. Moreover, there have probably been more trial failures in ALS than almost any other disease. It is a highly complicated and poorly understood disease and indeed there may be a number of different diseases that are lumped together under the ALS rubric. Expectations for any drug being developed for ALS must be muted.
The second paragraph of the press release reinforced this negative view as it said “FORTITUDE-ALS did not achieve statistical significance for a pre-specified dose-response relationship in its primary endpoint of change from baseline in slow vital capacity (SVC) after 12 weeks of dosing (p=0.11). Similar analyses of secondary endpoints of ALSFRS-R and slope of the Muscle Strength Mega-Score yielded p-values of 0.09 and 0.31, respectively. With no statistical significance in any of these endpoints, this seemed to be game, set and match to ALS.
A Closer Reading Suggests There are Reasons for Hope
As I read further, I flip flopped on my first reaction and came to a more positive view which was reinforced by a conference call with key opinion leaders on Monday morning. Let me first note that this was a phase 2 trial and the purpose of a phase 2 is to get an insight into as many factors as possible to determine whether to undertake a phase 3 trial and if so, how to design it. These include:
- Determining a medically important phase 3 endpoint(s) and secondary endpoints,
- Identifying the characteristics of patients who are most likely to benefit
- Dose ranging effect- efficacy should increase or be maintained as dosage is increased,
- Dose limiting side effects,
- The optimum dose that balances efficacy and side effects,
- Duration of the trial needed to demonstrate efficacy/ meaningful medical benefit,
- Is a therapeutic benefit maintained over a medically important time frame
- A broad range of other pharmacokinetic and pharmacodynamic data points
During the Monday call, CYTK had a panel discussion with three key opinion leaders, all of whom were positive on the trial results and recommended that CYTK progress reldesemtiv to a phase 3 trial. One KOL summed up the thoughts of the panel when she urged everyone to ignore the non-significant p=0.11 on the primary endpoint. Instead, she suggested that the results were really quite encouraging as one digs deeper into the data and sees strong evidence of a medically meaning effect on ALS, a positive dose ranging effect and a meaningful duration of effect.
Dr. Jeremy Shefner, the lead investigator on FORTITUDE-ALS summed up his positive view on the drug. He said that he had participated in or was familiar with research on almost all of the ALS drugs that have been studied. He said that in his opinion the FORTITUDE-ALS phase 2 data was the most positive of any of these drugs, including the only two approved drugs Riluzole (approved in 1995) and edaverone (approved in 2017).
Key Points of Press Release
-
- The trial showed effects favoring reldesemtiv across all three dose levels although statistical significance was not reached on the primary endpoint of SVC and the secondary endpoint of ALSFRS-r.
- Clinically meaningful differences between reldesemtiv and placebo in SVC and ALSFRS-r observed after 12 weeks of treatment were still evident at follow-up, four weeks after the last dose of study drug was given.
- Shefner said that it was especially noteworthy that there was consistency and durability of effects on clinically meaningful endpoints for each of the three doses of reldesemtiv used in the trial.
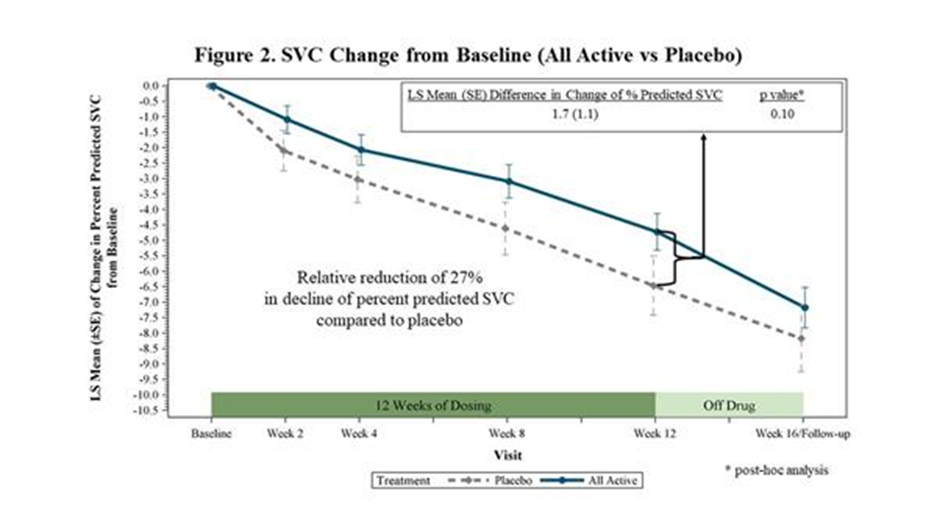
- The rate of decline in SVC in the placebo group was slower than has been observed in previous trials. FORTITUDE-ALS was powered with the expectation that patients receiving placebo would decline approximately 8.0 % during the 12-week trial. The actual decline was 6.5% so the trial was underpowered to show statistical significance.
- Angela Genge, a member of the trial's steering committee said this difference could be explained by the wide range of patients accepted for the study, which resulted in a meaningful number of slower progressing patients being enrolledl. The trial which led to the approval of edaverone enrolled patients who were progressing more rapidly. In a phase 3 trial, CYTK and Astellas might follow the same patient enrollment strategy which would be expected to increase the rate of decline in SVC for placebo patients.
- In a post-hoc analysis, when all active treatment groups were combined and compared to placebo, the trial showed a 27% reduction in the decline of SVC (p=0.10). This was close to stsatistical significance raising the strong possibility that enrolling more rapidly progressing patients and/or increasing the statistical power of the trial could lead to a positive outcome.
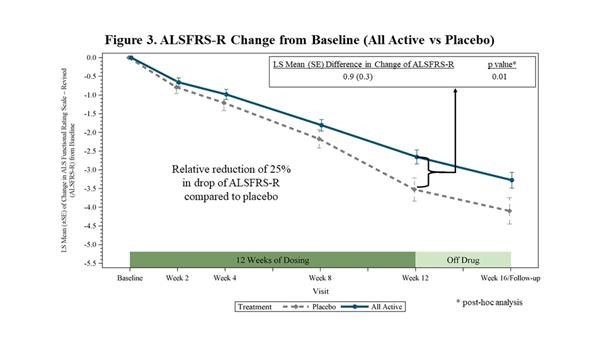
- The post-hoc analysis demonstrated a decrease in the decline of the ALSFRS-r from baseline to 12 weeks of 25% (p=0.01) when all active treatment groups combined were compared to placebo.
- If verified in a phase 3 trial, the 25% reduction would be a clearly clinically significant slowing in ALS progression. According to a 2010 survey of ALS clinicians conducted by the Northeast ALS Consortium (NEALS), respondents deemed a therapy that resulted in a change of 20-25% or greater in the slope of decline of ALSFRS-r to be a clinically meaningful effect.
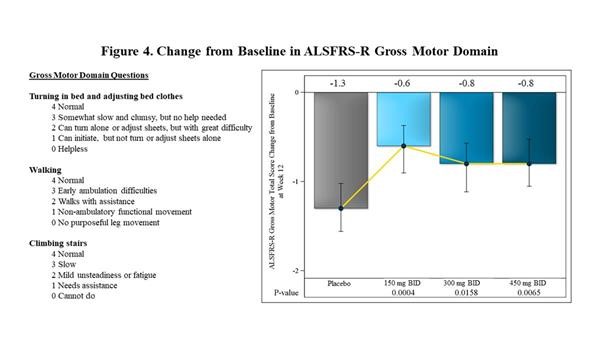
- Within the 12 different domains that comprise the ALSFRS-r scale, the largest effects observed at 12 weeks in FORTITUDE-ALS were in the Gross Motor Domain. This measures the ability of patients with ALS to turn in bed, walk and climb stairs. The effect of reldesemtiv was statistically significant at each dose vs. placebo (p=0.0004 for the 150 mg BID dose, p=0.0158 for 300 mg BID, p=0.0065 for 450 mg BID).
- The most common clinical adverse effects for reldesemtiv in the trial included fatigue, nausea and headache.
- The incidence of early treatment discontinuations, serious adverse events and clinical adverse events in FORTITUDE-ALS were similar between placebo and active treatment arms.
- The leading cause for early termination for patients who received placebo was progressive disease; the leading cause for early termination for patients who received reldesemtiv was a decline in cystatin C based estimated glomerular filtration rate, a measure of renal function. The effect on glomerular filtration was reversed when the drug was discontinued. Because of this, the key opinion leaders thought that this would not dissuade doctors and patients from trying the drug.
Tagged as Cytokinetics, FORTITUDE-ALS Trial Results + Categorized as Company Reports, LinkedIn