ImmunoCellular Thoughts on Potential Outcomes for the Phase II Trial of ICT-107 in Newly Diagnosed Glioblastoma (IMUC, $2.31) (Subscribers Only)
Investment Viewpoint
ImmunoCellular has extremely important data upcoming on the phase II trial of ICT-107 in newly diagnosed glioblastoma; topline results are anticipated in 4Q, 2013 or 1Q, 2014. There are four biotechnology companies- ImmunoCellular Therapeutics (IMUC), Northwest Biotherapeutics (NWBO), Agenus (AGEN) and Celldex (CLDX)-that currently are in phase IIb and III trials of cancer vaccines for newly diagnosed or recurrent glioblastoma. Earlier phase I and II trials for each company have reported data that has been impressive, but it has been largely dismissed by investors because the trials were small and not randomized. Critics also maintain that patients enrolled in these trials may have been healthier patients who were more likely to have positive outcomes than the broad glioblastoma population.
This 124 patient randomized trial will be the first high quality trial conduced in this disease setting with a cancer vaccine and will address the issues laid out by critics in a meaningful way. The phase I data for ICT-107 leads me to believe that there is a therapeutic effect that isn’t just due to selection of patients. For example 3 of 16 patients or 19% were alive at five years which compares to about 4% for standard of care. Moreover median overall survival was 38.4 months which compares to 18.1 months which is generally accepted as the probable outcome with standard of care. The claim that patients in the phase I trial were more fully, surgically resected is the most frequent reason cited by critics for why ICT-107 results were so strong.
The most positive trial outcome would be that ICT-107 hits its primary endpoint and shows an 8.0 month increase in median overall survival versus the 18.1 months expected for the control arm; the effect on the stock would be profound. Generally a 4.0 to 5.0 month increase in an aggressive cancer like glioblastoma would be viewed as a major clinical advance. With the mild side effect profile seen with this drug, the clinical benefit would be magnified through greatly improved quality of life. I would not be surprised to see the stock quadruple to $10.00 with such data. In the event that the data is disappointing, there would be a devastating effect on the stock as its whole dendritic cell technology would come under question. I could see the stock below $0.50 in this outcome.
Based on the very strong results seen in phase I, I would be shocked if ICT-107 does not show some therapeutic benefit relative to control. Investors should consider that this may not be a binary event. There is the possibility that this trial could fail to reach its primary endpoint and still allow the company to define a path forward. For example, I think that a 4.0 month increase in median overall survival might be sufficiently encouraging to begin a phase III trial. It might also be possible to define sub-groups who benefit most and could be the basis for a phase III trial, though benefit for the broad population is not shown. Management has discussed such possibilities in each of the last two quarterly conference calls.
I have learned from long experience that predicting trial outcomes is hazardous. My best judgment is that the primary endpoint won’t be reached but that the data will still warrant conducting a phase III trial. This is not a judgment based on objective data. I do buy into the thesis that the patients in phase I for ICT-107 were healthier than those that were enrolled in this phase II trial so that results will be less robust. I also think that the primary endpoint calling for an 8.0 months improvement in median overall survival (something like 26 months) is a very high hurdle to jump over.
I think that the initial reaction to an announcement that the trial failed to meet its primary endpoint would cause a swift decline in the stock. There are a substantial number of investors that I call the “Twitter traders” who write and think in terms of 140 characters and will not try to understand the entirety of the data. A major purpose of this note is to prepare readers for this possibility which I view as the most likely outcome; it could result in a major buying opportunity. Remember that my most likely case is that the trial will not reach its primary endpoint, but will show a path forward for doing a phase III trial. Of course, all of this is data dependent. This would be a strong positive for ImmunoCellular and the whole field of cancer vaccines. It would suggest that cancer vaccines are a new and viable option for treating cancer and that IMUC would be at the forefront of this.
I have seen some reports from a few investors who in contrast to the widespread pessimism of Wall Street types believe that the phase II data will be so good that the Company can file for regulatory. Current management has downplayed this possibility, but prior management had been more upbeat on the possibility. I hope that the trial does reach its primary endpoint as this would be good for patients and investors. However, in this event I take the point of view that the Company could probably not file for approval and would have to do a phase III trial.
The phase II trial was conducted at two different manufacturing sites and while the process at each was similar, it was different. The FDA in addition to requiring that clinical data be convincing for regulatory approval, requires that the manufacturing process used in clinical trials be the same as that which will be used for commercialization. This is to assure that the products are manufactured in a rigorous way that assures that the product proven effective in clinical trials is the same as that sold commercially. IMUC has not yet settled on the manufacturing process that will be used in phase III. Because of this, I believe that even in the event that the phase II trial meets its endpoint that IMUC will be required to do a new phase III trial in which the manufacturing process is validated.
So what does one do with the stock? In my most likely scenario, I see the trial as not reaching its primary endpoint and resulting in a sell off led by the Twitter traders. I own a small position in the stock on the basis that I think there is much greater chance of the trial reaching its primary endpoint and perhaps quadrupling if my judgment is correct as opposed to complete failure that ends the development of ICT-107 and destroys much of the stock price. I want some exposure to this possibility. I think that the most likely outcome is an in between scenario that points to a path forward, but could have a short term negative effect due to the Twitter traders. This could be the point at which I might significantly increase my position in the stock. Obviously, this is dependent on the data.
Why I Am Interested in ImmunoCellular
I have a keen interest in cancer vaccines that are being developed by four emerging biotechnology companies to treat glioblastoma. ImmunoCellular Therapeutics and Northwest Biotherapeutics use a technology based on loading the patient’s own dendritic cells with cancer antigens. Agenus uses a technology based on heat shock proteins. Celldex’ rindopepimut uses a still different approach that is based on combining a peptide fragment that mimics EGFRvIII that is conjugated with KLH. Each of these products in small open label studies has produced impressive results. However, these have been largely dismissed by investors as being small and flawed trials. Besides critics add, everyone knows that cancer vaccines don’t work as can be seen in a long string of cancer vaccine development failures.
In regard to cancer vaccines for glioblastoma, there are two important data sets coming up that should give us a better insight into whether the promising signals of activity that we have seen are validated in subsequent studies or if the critics are right and cancer vaccines don’t work. Results for 25 patients with recurrent glioblastoma who were treated with rindopepimut will be reported at the Society for Neuro-Oncology Annual Meeting of November 21 through 24 in San Francisco. The abstract of the presentation was available on the SNO website on Monday, November 11, but was not that informative. The abstract will be presented in an oral presentation during the conference and is likely to present some more meaningful data. More important is the topline data readout on the 124 patient phases II trial of IMUC’s ICT-107 in newly diagnosed glioblastoma which should occur late this year or early 2014.
How Do the Four Cancer Vaccines Compare?
Let me anticipate your next question as to how the four vaccines under development compare with each other and how they will be used in clinical practice? The next table contains some of the relevant data on the four cancer vaccines in development.
|
Clinical Trial Data For Cancer Vaccines for Newly Diagnosed Glioblastoma |
||||
| Drug | Patients Treated | Median Overall Survival for Drug (months) | Median Overall Survival for Standard of Care (months) | Difference in Median Overall Survival (months) |
| rindopepimut |
65 |
21.8 |
16.0 |
5.8 |
| DCVax-L |
20 |
36.0 |
18.1 |
17.9 |
| ICT-107 |
16 |
38.4 |
18.1 |
20.3 |
| Prophage |
46 |
23.3 |
18.1 |
5.2 |
| Standard of care |
287 |
14.4 |
14.4 |
** |
There is a very hot debate on the interpretation of the numbers in the above table because none of these trials were randomized. This means that results can only be compared to results from another trial done at a different time and place and with a different patient population. With justification, critics point out those patient populations of different trials can vary widely in their characteristics so that comparing results in one trial to another can be misleading. The defining trial for standard of care in glioblastoma is the Stupp trial that defined temozolomide plus radiation as standard of care in newly diagnosed glioblastoma. The median overall survival in the Stupp trial was 14.4 months.
Comparing results from the Stupp trial to these smaller trials can potentially be misleading. Stupp accepted patients of varying physical condition while those patients included in the four above trials were likelier to be more carefully selected, healthier and would be expected to have better outcomes. There have been particular questions raised because patients in these smaller trials may have had their tumors more fully surgically resected than those in in the Stupp trial which could lead to a better outcome. All of these criticisms are valid but it still seems to me that we are seeing strong signals of activity.
I have tried to adjust the Stupp trial data in a more conservative fashion. I used data from a subset of the Stupp trial that was made up of 91 patients who were greater than 90% surgically resected. The median overall survival in this group was 18.1 months as opposed to the 14.4 months for the entire trial. In the above table, I compare the median overall survival of DCVax-L, ICT-107 and Prophage to the 18.1 months seen in this subset. I compare rindopepimut to median overall survival seen in a retrospective study of patients with the EGFRvIII mutation that was done by M.D. Anderson.
ICT-107 can only be used in HLA A1/A2 positive patients which were about 45% of patients screened for its phase II trial. The mode of action of rindopepimut means that it can only be used in patients who are EGFRvIII positive which is about 30% of patients. DCVax-L can be used in all patients with sufficient tumor mass after surgery to manufacture the product; this is over 95% of patients. Prophage can be used in virtually all patients. It may be the case that these vaccines could be used in combination or in sequence.
More Detail on ICT-107 Phase I Results
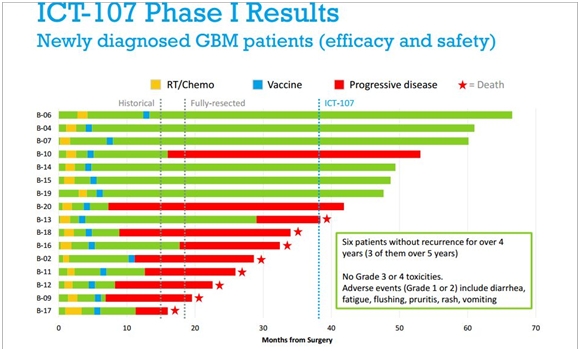
The next table is a waterfall plot showing individual patient results for the phase I trial of 16 patients with newly diagnosed glioblastoma treated with ICT-107. This data was last presented in October of 2012 and has not been updated since.
There is a lot of information in this one table. The data starts from the time of surgical resection at month 0. The yellow box then notes the start of radiation and temozolomide which is standard of care. The blue box denotes the start of ICT-107 vaccinations. As long as the line remains green, the patient is alive without progression of the disease. As it turns from green to red, it means that the disease has returned and the cancer is progressing. A red star denotes that the patient has died.
Source: ImmunoCellular Investor Presentation, October 2012
There is a dotted vertical line at 14.4 months which represents the median overall survival in the Stupp trial. There is another dotted line at 18.1 months which is the median overall survival for a sub-group of the Stupp trial which was made up of patients who had been fully surgically resected. Remember that one of the criticisms directed against this data is that patients were more surgically resected than in the Stupp trial and have better survival prospects. Finally there is a dotted vertical line at 38.4 months which is the median overall survival for the 16 patients with newly diagnosed glioblastoma in the trial.
As highlighted in the box, six of 16 patients were alive without progression for over four years and three without progression for over five years. The bears make the argument that this trial only selected the healthiest patients with the best prognosis for survival. To my knowledge, the patient characteristics that would allow an understanding of each of these patients in the trial have not been published to allow a more precise determination of this issue.
Given the aggressiveness and rapid progression of glioblastoma, these results seem to me to be quite impressive even if the trial was heavily weighted toward patients with the best potential for a good outcome. The National Brain Tumor Society estimates that only 4% of newly diagnosed glioblastoma patients survival five years. In the ICT-107 trial, 19% survived for five years. It is also important to note that unlike chemotherapy and targeted therapy, the side effect profile appears relatively benign.
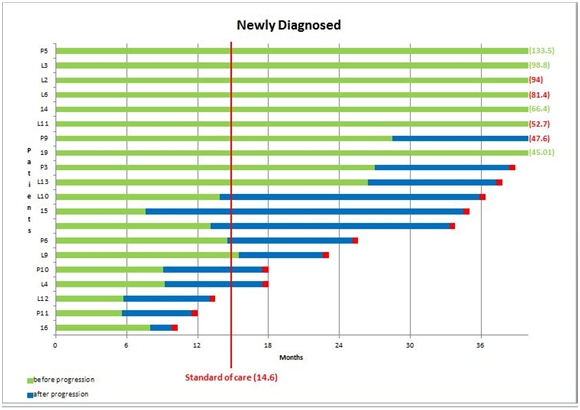
In the next table, I show a waterfall plot for 20 newly diagnosed glioblastoma patients treated with Northwest Biotherapeutics DCVax-L. The graphical presentation is again a waterfall plot that is set up in a slightly different way. However, what is striking is that the outcomes are very comparable to ICT-107.
DCVax-L Phase I Results for Newly Diagnosed Glioblastoma Patients
Source: Northwest Biotherapeutics Corporate Presentation
The green line represents the period of time before the disease begins to recur. The blue line represents progression of the disease and the red box represents death. The red vertical line is the 14.4 months median overall survival seen in the Stupp trial. The median overall survival is 36.0 months.
Like ICT-107there were some remarkably long survivors. Six of 20 patients were progression free at about four years or more. The best progression free survival was 135.5 months or over 11 years and five patients or 20% were progression free at five years. This is comparable to the 19% seen with ICT-107 and compares to an expected five year survival rate of 4%
ICT-107 and DCVax-L are both based on autologous dendritic cells. These cells are loaded with tumor antigens in different ways. ICT-107 uses six peptide mimics of antigens that most frequently occur on glioblastoma cells. DC-Vax-L lyses the tumor with the goal of loading dendritic cells with all antigens specific to the patient’s own tumor. The similarity is the use of dendritic cells and it is striking to me that the results for ICT-107 and DCVax-L are pretty similar. I can hypothesize that the results of each trial validate the other.
Results for Phase II Trial of ICT-107 are Imminent
ImmunoCellular began a phase II trial of ICT-107 in 2011. This phase II trial was based on 124 patients with an endpoint of median overall survival. It was randomized roughly 2:1 so that about 83 patients received ICT-107 plus standard of care and 41 received placebo plus standard of care. The statistical powering of the trial assumes an improvement for the ICT-107 regimen of 8.0 months over 18.8 months median overall survival assumed for standard of care.
The study will be unblinded and the data analyzed when the 64th patient in the trial dies. This event should occur sometime in the 3Q, 2013 or 4Q, 2013 but this will not be announced to investors. It will take a few weeks to break the blind and analyze the data. Management anticipates that they can release topline data in 4Q, 2013 or 1Q, 2014. Of course, management is blinded to the data until the time of the topline release.
My excitement with ICT-107 and that of many other investors comes from the very long survival tails that were seen in the 16 patient phase I trial in which median overall survival was 38.4 months and in which 55% of patients survived for more than three years. The phase II trial that was started in 2011 cannot be expected to provide the same insight into long term survival since perhaps more than half of patients were enrolled after mid-2012 and have only been on the drug for a year and one-half or so.
However, if the trial hits its end point of an 8.0 month improvement in median overall survival, it would be a pretty spectacular achievement as oncologists generally consider a 4.0 to 5.0 month increase in median overall survival in a rapidly growing cancer like glioblastoma multiforme as a major advance. Temozolomide, which is the oncology drug used in standard of care for glioblastoma, was approved on the basis of a 2.5 months median overall survival increase. Zytiga and Xtandi, which are on their way to being $2+ billion blockbusters, were approved on the basis of about 4.5 months of median overall survival in metastatic prostate cancer.
What Comes After Phase II Results Are Reported?
There is a broad spectrum of possible outcomes for the phase II trial ranging from achieving the primary endpoint of an increase in median overall survival of 8.0 months to no discernible effect. I have many times been humbled by trying to predict the outcome of a clinical trial. The results in the phase I trial persuade me that there is a therapeutic effect and I would be shocked to see no benefit when compared to standard of care. However, I would not be shocked nor necessarily alarmed if the trial did not reach what seems to me to be an aggressive primary endpoint.
If the study reaches its primary endpoint, IMUC would have a clear path forward to phase III, but what if the trial does not reach statistical significance on the primary endpoint. I think that if the trial shows median overall survival of 4.0 months or possibly less, it would justify doing a phase III trial. There will also be analyses done on various sub-groups within the trial as determined by factors such as HLA1 or HLA 2 status, MGMT status, age, gender, extent of surgical resection. Even if the trial as a whole is unsuccessful, these sub-group analyses could point the way forward in a more narrowly defined sub-group.
There is hope on the part of some investors that the phase II data will be so positive that IMUC will file for approval in the US and will not need to conduct a phase III trial. The current CEO has not taken this position as he consistently has stated that he believes that the Company will have to do a phase III trial; however, his predecessor did not discourage thinking that the Company might file on the basis on phase II data. I think that there is virtually no chance that the Company can file for approval even in the event that the phase II trial meets its primary endpoint because of manufacturing issues.
ICT-107 production is based on the maturation of monocytes obtained from a patient through a blood draw that are then matured into dendritic cells, loaded with tumor antigen and re-injected in the body. IMUC conducted its phase II trial using product supplied from two different GMP production facilities, first at the University of Pennsylvania and later at a NeoStem (NBS) facility. Manufacturing for phase I was done at Cedars Sinai in Los Angeles. The FDA has a stringent requirement that the manufacturing process and quality control assays used in clinical trials be nearly identical to those which will be used when the product is commercialized. This should be especially true for living cell therapy products in which the manufacturing process is essentially the product. The FDA has to be concerned that there may be differences in the products that were manufactured at the two sites that could meaningfully affect product characteristics.
The phase II trial was done with two different manufacturing processes and the Company is planning on a third for commercialization. The differences between these processes may not be significant, but how can the FDA make such a decision without a phase III trial in which the manufacturing process is validated? I believe that this issue will cause the FDA to ask for a phase III trial that uses the manufacturing process that will be used in both the phase III trial and then for commercialization, even if the phase II trial reaches its primary endpoint.
The base case scenario that management is looking at is a phase III trial. If the results in the phase II result in a decision to move to phase III, the first step would be an end of phase II meeting that could occur in mid-2014 to discuss the potential phase III trial design with the FDA. After that, the Company could move fairly quickly into selecting sites for the phase III trial.
At each site, it might take about 6 months to get approval from its IRB to conduct the trial. This suggests that the phase III trial could actually start enrolling in late 2014 or early 2015. The phase II trial started enrolling in February 2011 and the topline data readout is about three years later. With a similar timeline for ICT-107, the data readout on ICT-107 would be in early 2018.
They also will probably need to build a manufacturing facility(s) in European countries. ICT-107 is an autologous vaccine which means that IMUC collects the patient’s own monocytes through a blood draw. These cells are then shipped to a manufacturing site at which they are matured into dendritic cell precursors and are loaded with six peptides that mimic antigens that are highly expressed in glioblastoma. The finished product is frozen and can be stored at the manufacturing site or shipped to the treatment center. Shipping through customs can create significant issues so that having manufacturing sites in specific customs areas is greatly preferred. This is the model that Northwest Biotherapeutics has followed in setting up manufacturing facilities in the UK and Germany.
Tagged as clinical trials, glioblastoma, ImmunoCellular Therapeutics LTD, IMUC, phase II + Categorized as Company Reports, Smith On Stocks Blog