Discovery Laboratories: An Analysis of Phase 2a Results for Aerosurf and What to do with the Stock (DSCO, $0.81, for paid subscribers)
Glossary of Important Definitions
For those of you who are not familiar with terms used in neonatology, I am starting this report with some definitions used in this report to explain the outcome of the recent phase 2a trial of Aerosurf.
Surfactant
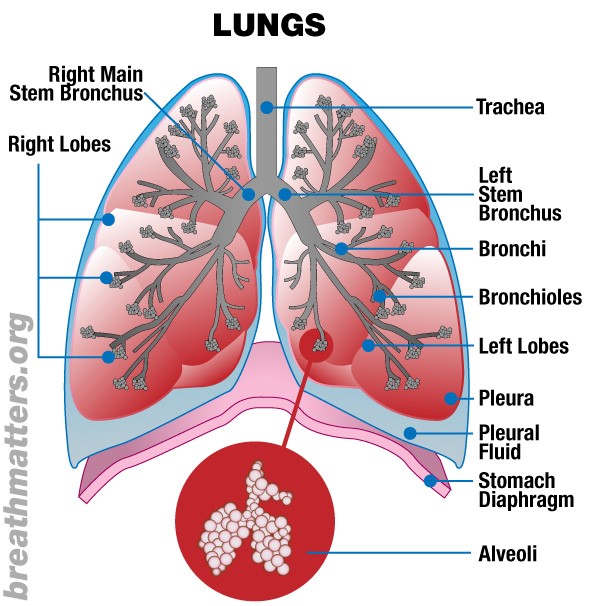
Surfactant coats the alveoli (the air sacs in the lungs where oxygen enters the body); it is a mixture of lipids (fats) and proteins. Surfactant reduces surface tension in the alveoli which makes it easier for them to expand and remain open during breathing. This makes it easier to oxygen into the blood and to get carbon dioxide out. Think of the lung as a balloon that surfactant makes easier to blow up.
Respiratory Distress Syndrome (RDS)
RDS is a lung injury that is caused by an inadequate amount of surfactant, a condition which occurs in many premature infants born before their lungs are mature enough to make adequate amount of surfactant. Low amounts of surfactant lead to poor lung function resulting in stiff, hard to inflate lungs and increased fluid in the lungs; this makes for difficult breathing. Babies born with this condition have a bluish instead of pinkish color and can be referred to as “blue babes”.
Bronchopulmonary Dysplasia
Babies who are born very prematurely and develop RDS are at risk for bronchopulmonary dysplasia (BPD). Dysplasia means abnormal changes in the structure or organization of a group of cells. The cell changes in BPD take place in the smaller airways and lung alveoli, making breathing difficult and causing problems with lung function. Most infants recover fully but sometimes BPD can be serious and require intensive medical care. Babies aren't born with BPD; it develops when premature infants with RDS need help to breathe for an extended period. It can lead to scarring in the lungs and other troublesome complications.
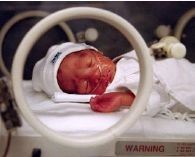
Nasal Continuous Airway Pressure (nCPAP)
Nasal continuous positive airway pressure (nCPAP) is the application of positive pressure throughout the respiratory cycle to the airways of neonates capable of breathing on their own. nCPAP can in some cases be a relatively simple and effective therapy for RDS that can establish and maintain lung volume. nCPAP is delivered through nasal clips as shown below.
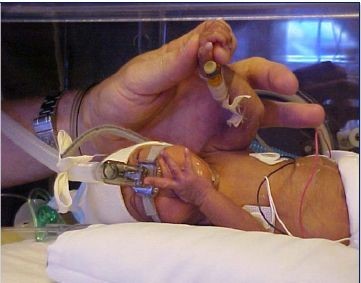
Mechanical Ventilation
Mechanical ventilation is used to mechanically assist or replace spontaneous breathing with a ventilator. It requires the insertion of an endotracheal tube through the mouth, the trachea and then into the lungs through which positive pressure is used to deliver a gas breathing mixture. Positive pressure causes the gas to flow into the lungs until the ventilator breath is terminated. As the airway pressure drops to zero, elastic recoil of the chest accomplishes passive exhalation by pushing the tidal volume out. The endotracheal tube used in mechanical ventilation is also used to deliver liquid, animal derived surfactants for babies with surfactant deficiencies. This procedure is much more intrusive than nCPAP; you can see the endotracheal tube that is inserted in the mouth and is connected to a mechanical ventilator.
Surfaxin
This is a liquid form of the first non-animal based surfactant KL-4. Animal surfactants are salvaged from the lungs of swine and cattle. It has been approved by the FDA, but the launch was a failure and marketing efforts have been suspended.
Aerosurf
Aerosurf delivers KL-4 surfactant which has been approved by the FDA as a liquid surfactant and was marketed for a time as Surfaxin. Aerosurf surfactant delivery technology starts with a lyophilized form of KL-4. A proprietary and highly sophisticated aerosol generator then delivers a high output steady stream of the aerosolized form of KL-4 to the lungs via the nCPAP system. The goal is to deliver effective amounts of surfactant to the lungs with the same physical properties as liquid surfactants. If this can be done safely and effectively, it would be a major medical advance that would transform the management of RDS in the neonatal intensive care unit (NICU).
FiO2
In interpreting the results of the phase 2a study of Aerosurf, it is important to understand the definition of FiO2. This is simply a measurement of the amount of oxygen that someone is breathing. The air around us is 78.1% nitrogen, 20.9% oxygen, 0.9% argon and 0.1% carbon dioxide and other gases. Therefore, the FiO2 for people breathing normal air is 0.21 (21%). If someone in the hospital is on a breathing machine that is delivering pure 100% oxygen, FiO2 would be 1.00 (100%). FiO2 stands for fraction of inspired oxygen.
CO2
Measurement of the partial pressure of CO2 in the blood provides a measure as to whether the lungs are effectively removing CO2 from the blood
Gestational Age
Gestational age is the common term used to describe how far along a pregnancy is. It is measured from the first day of the woman's last menstrual cycle to the current date. A normal pregnancy is usually 40 weeks. The first day of the menstrual cycle is the first day of a woman’s period which lasts 3 to 7 days.
Aerosurf Is a Unique Product in Biotechnology
The investment case for Discovery Laboratories rests totally with Aerosurf which I believe to be one of the most unique and promising product development opportunities in biotechnology. It potentially addresses a great unmet medical need in neonatal intensive care by allowing much easier and broader use of surfactant replacement therapy in premature babies with RDS. This could provide very meaningful survival and morbidity benefits in the premature baby population
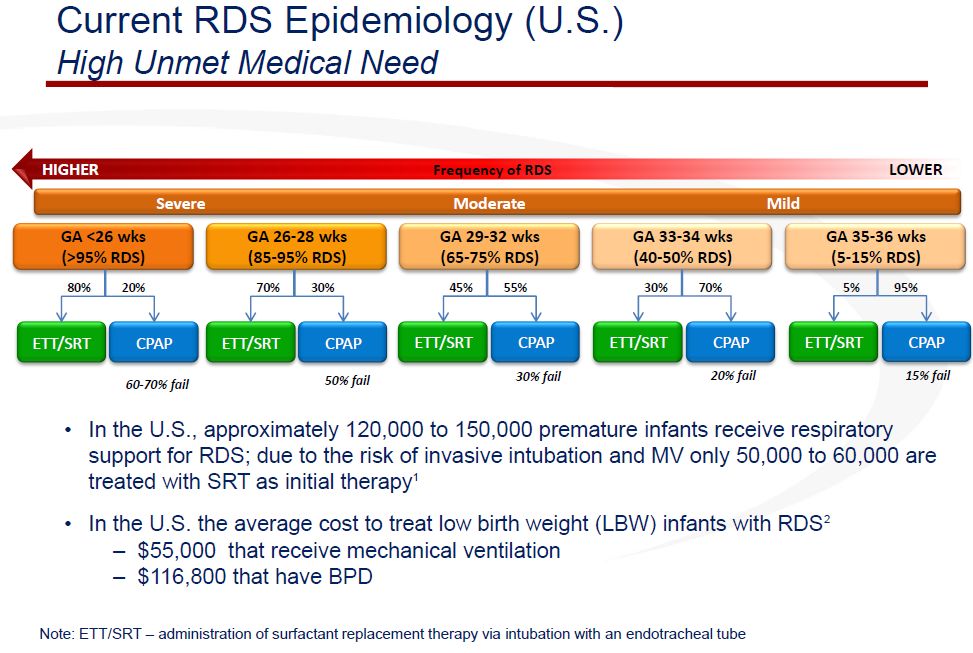
Addressable Market is Large and No Competition is on the Horizon
Somewhere between 120,000 and 150,000 premature babies require respiratory support in the US annually to treat or prevent RDS. Roughly half of these are initially intubated, put on a mechanical ventilator and given surfactant. Treating babies who develop RDS costs about $55,000 and if they develop bronchopulmonary dysplasia it is even greater at $167,000. The primary population for Aerosurf is babies between 24 and 36 weeks who are started on nCPAP. This is a market of about 60,000 to 75,000 babies annually in the US.
Management has not given guidance on the potential price for Aerosurf, but the most frequent estimates of price have been about $8,000 per course of therapy. If so, the annual addressable US market for Aerosurf is $480 to $600 million. The European market is about the same as the US and the market in remaining parts of the world is about half of the US indicating a global addressable market of $1.2 to $1.5 billion.
I am not aware of any viable competitive drugs in advanced development. Because of the great unmet medical need and the promise of a strong benefit to risk ratio and favorable pharmacoeconomics, I would expect Aerosurf to have a swift uptake if it comes to market-unlike Surfaxin- and to penetrate 60% of the market in a four year time.
Aerosurf has very unique and desirable characteristics for a pipeline product. In many other drug categories, most notably oncology, there are seemingly an endless number of new products in development. An investor has to be concerned not only that the product is safe and effective, but also has to make a decision on how it will fare against competitive products. With Aerosurf, the concern is only if it is safe and effective because of its uniqueness. The other impressive potential of the product is that it can be lifesaving and result in the baby leading a normal life or prevent diseases which can have a lifelong negative effect on its lungs; the survival benefit can be a lifetime. Contrast this to oncology in which a 4 to 5 months increase in survival for a human at the end of their life is considered a major advance.
Potential for Successful Development of Aerosurf
Aerosurf carries a lesser development risk than a drug based on a new molecular entity. The active drug component KL-4, is the same as Surfaxin, which has been shown to be safe and effective in clinical trials and was approved by the FDA. We know the drug works. Similarly, the manufacturing process has been validated; problems with manufacturing sometimes lead to long delays as was the case with Surfaxin.
I should point out that Aerosurf is a drug combined with a novel medical device that aerosolizes KL-4 and delivers it to the lungs. The key uncertainty is whether Aerosurf can effectively disperse the active KL-4 ingredient throughout the lungs. The company licensed in a device called a capillary aerosol generator nearly ten years ago and has refined the device significantly. Studies in the lungs of lambs, which are a good animal model, have shown that Aerosurf can deliver a therapeutically effective dose of aerosolized KL-4. The phase 2a trial of Aerosurf which is the subject of this report is the first use of Aerosurf and test of the capillary aerosol generator in human babies.
The primary goal of the phase 2a trial was to show that the drug is safe and that the capillary aerosol generator used to deliver Aerosurf is working as intended and is user friendly in the neonatology intensive care unit. Both objectives were met and Aerosurf will begin a phase 2b proof of concept trial in 4Q, 2015 or 1Q, 2016. The phase 2a was a small study in which only 24 infants received Aerosurf; there were 8 patients on each of three doses. Also, only one dose was given per patient. There were indications that Aerosurf was achieving a short term effect. Over a 3 hour period Aerosurf treated babies required less oxygen support than control babies and had reduced levels of CO2 in their blood. This is an indication that Aerosurf was having an effect that would be expected of a surfactant.
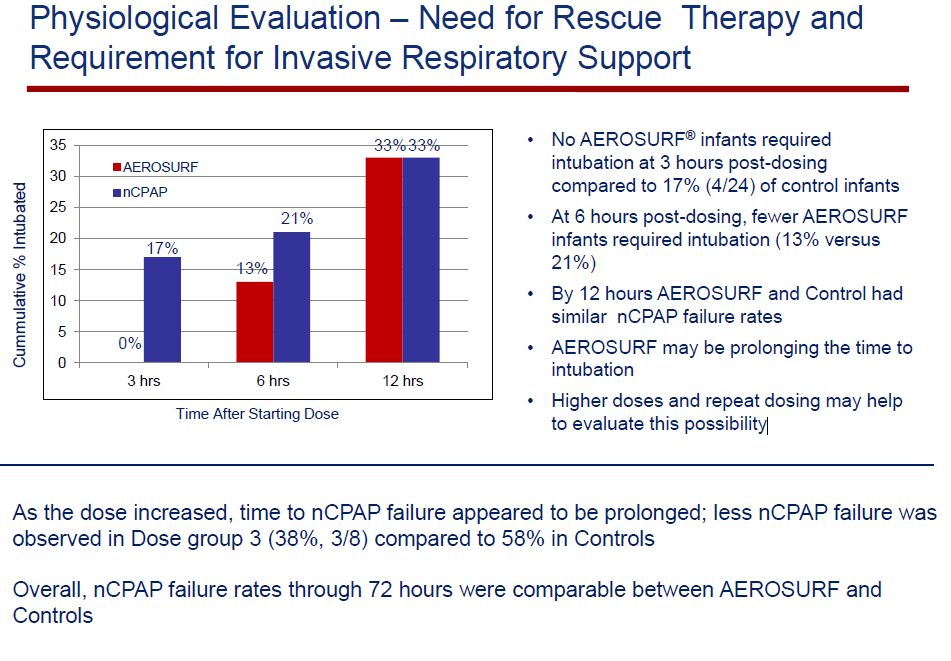
The therapeutic goal of Aerosurf is to reduce the number of babies treated with nCPAP who then have to be intubated and mechanically ventilated. The study looked at nCPAP failures and the need for reintubation over 12 hours. No Aerosurf treated babies required reintubation at three hours post dose compared to 17% of controls. At 6 hours post dose, 13% of Aerosurf babies required intubation versus 21% of controls. However, at 12 hours, the rate of failure was the same at 33%. This supports the hypothesis that Aerosurf is effective, but only for a short period of time. The hope is that by giving larger doses or repeat doses a sustained clinical effect can be achieved. This also has to be done with no safety issues.
Putting odds on success of phase 2b is difficult. However, the preliminary information coming out of phase 2a on safety and tolerability along with animal studies makes me hopeful that Aerosurf will be the success we are all hoping for. The phase 2b trial could complete in 3Q, 2016 and issue topline results in 4Q, 2016. Success in this trial would be an enormous catalyst which I think could lead to a market capitalization in the $500 to $750 million range. The Company could probably then go into a confirmatory phase 3 trial that could complete in early 2018. If successful, approval could be received in late 2019.
The Dark Side of Discovery Laboratories
I would submit that if Discovery were a private company coming public in the current hot market for biotech IPOs it would be priced at a market capitalization of around $1 billion. It currently has a market valuation of $92 million due to baggage accumulated over the past decade. I have been involved with this company for a number of years and so far it has been my worst single investment in biotechnology (so far) and the same is true for many others. Almost every investor who has bought and held the stock over the past twelve years has lost money as the price declined from $8.14 on July 9, 2003 to the current price of $0.79.
The Company filed an NDA for Surfaxin on February 14, 2005, but did not receive approval until October 4, 2013. In between there was a heart breaking string of seven complete response letters in which the DSCO refiled the NDA and the FDA refused to approve the drug. Very importantly, the FDA had no questions on the safety and efficacy of the drug and the clinical trials which led to its ultimate approval. All of the refusals to approve were based on validation of a test used to assure quality of the product. The back and forth between the FDA and company was ultimately resolved without new trials and with clear communications might have been resolved in 2006, but they weren’t. The Company and the Agency both shared blame for this debacle.
At last, the NDA was approved on October 4, 2013 and Surfaxin was brought to market in early 2014. Management had guided to $50 million for peak sales in the US. However, one year later the product had not achieved cumulative sales of $1 million. This was probably because the very well established animal surfactants are quite effective and were much more difficult to replace in NICU practice than anticipated. Faced with limited cash resources, Discovery chose in April 2015 to focus all of its financial resources on Aerosurf and cease marketing of Surfaxin.
Even with Aerosurf there was an issue that penalized investors. The trial was originally supposed to produce topline results in 2Q, 2014. However, changes in protocols for the trial resulted in a change in guidance to 4Q, 2014. But true to form, the trial wasn’t completed and topline results weren’t released until 2Q, 2015.
To say that management has a credibility gap with investors is one of the great understatements in biotechnology investing. There is widespread investor concern that as has been the case consistently in the past something or somethings will go wrong with Aerosurf development. I can’t argue against this logic and at this point if you want to click off of my website, I totally understand.
Price Target Thinking
Share Count
Investors need to understand that the Company will probably have to raise about $30 million to get to 2H, 2016 at which time phase 2b results could be announced. The stock is selling at $0.81 per share and based on a comparison with other public deals, I think the Company might have to issue shares at a 10+% discount ($0.71 per share) and issue 75% warrant coverage at a strike price of a dollar. As of March 31, there were about 85.5 million issued shares, 15.5 million warrants outstanding, 6.6 million shares reserved for equity incentive programs and 6.1 million options. This totals 113 million potential shares. The probable equity deal as described could add 42 million shares and 32 million warrants leading to a potential fully diluted share count of 189 million shares in 2H, 2016.
One Year Price Target
As I previously said, I think that success in the phase 2b proof of concept trial in 2H, 2016 could lead to a $500 to $750 million market capitalization. Also, I think that the phase 2a trial is encouraging and signals that there is a reasonable possibility of success in phase 2b. We can then divide market capitalization by number of shares outstanding to get a one year price target of $2.65 to $4.00. If the Company for whatever reason stumbles again on the development of Aerosurf in the form of a bad trial result or another of its patented delays, the investor could be looking at a downside of $0.00.
I downgraded the stock late last year and then one week later sold my stock and took a tax loss. My inclination is to go back to a buy after the equity deal is completed. Some days thereafter, I will then repurchase the stock for my own account.
Treatment of RDS
Mild cases of RDS can be treated by oxygen supplementation that increases the level of FiO2 throughout the respiratory cycle. Remember that that room air has a FiO2 of 0.21 (21.0% is oxygen). Babies with mild RDS may be given FiO2 levels of 0.25 (25%) to 0.40 (40%) or even higher. However, higher FiO2 levels can be toxic so that the lower the FiO2 levels the better. A second key objective in treating RDS is to help the baby to open and keep open the lungs by using positive airways pressure.
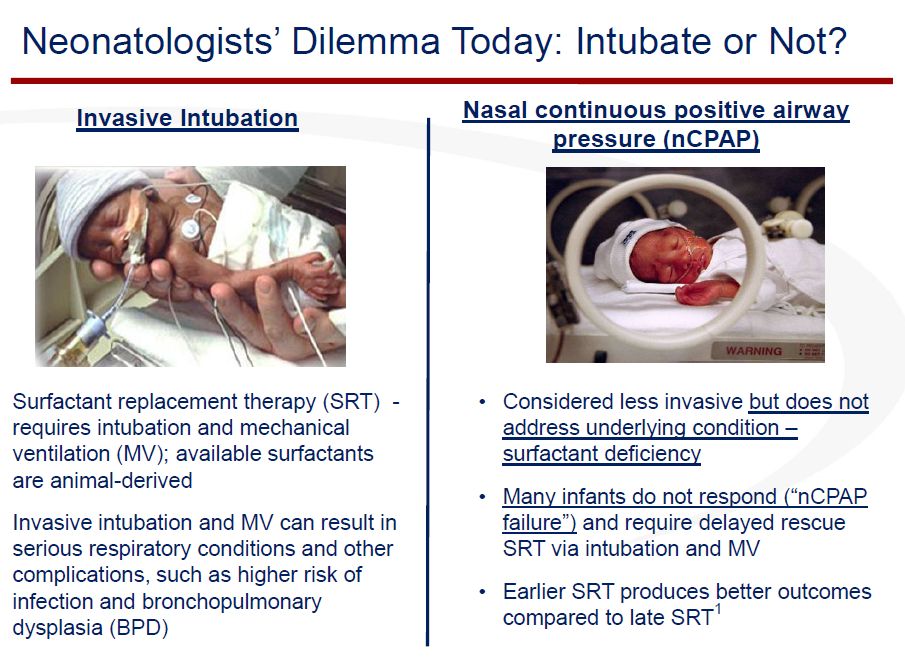
Two basic technologies are used primarily to treat RDS. Some babies can be treated initially with nCPAP while others require the more intrusive and potentially more dangerous mechanical ventilation. If at all possible doctors prefer to use nCPAP, but if the baby cannot be adequately treated, they turn to intubation and mechanical ventilation. Intubation is an invasive procedure in which a breathing tube is inserted through the mouth, the trachea and into the lungs.
With current technology, liquid surfactant can be administered through the endotracheal tube used in mechanical ventilation. However, there is no current method for delivering surfactant with nCPAP. This creates a dilemma for the neonatologist as to whether they should intubate, apply mechanical ventilation and give surfactant or avoid the risk of intubation and support the baby by the use of nCPAP. Intubation and mechanical ventilation can increase the risk of serious respiratory complications such as infection and bronchopulmonary dysplasia.
nCPAP is much less invasive and hence much safer, but it does not deliver surfactant. This is a serious issue as earlier surfactant administration leads to better outcomes. The unmet medical need addressed by Aerosurf is being able to deliver an aerosolized surfactant early in the course of RDS without intubation and mechanical ventilation and avoids their serious complication risks.
Incidence of Respiratory Distress Syndrome
The incidence of RDS varies inversely with gestational age. The incidence of RDS for babies of less than 24 weeks gestational age (remember normal is 40 weeks) is 90%. The incidence for babies of about 36 weeks is 5%. Intubation and mechanical ventilation is more commonly used in younger babies and nCPAP is more common in older babies. The success of nCPAP increases as babies get older. Physicians often choose to start with nCPAP even if there is a substantial risk of failure and if nCPAP fails they then intubate and use mechanical ventilation.
Somewhere between 120,000 and 150,000 babies require respiratory support in the US annually. Roughly half of these are initially intubated, put on a mechanical ventilator and given surfactant. Treating babies with RDS costs about $55,000 and if they develop bronchopulmonary dysplasia it is even greater at $167,000. The primary population for Aerosurf is babies between 24 and 36 weeks who are started on nCPAP. This is a market of about 60,000 to 75,000 babies.
Management has not given guidance on the potential price for Aerosurf, but the most frequent estimates of price have been about $8,000 per course of therapy. If so the addressable US market for Aerosurf is $480 to $600 million. I am not aware of any viable competitive drugs in development.
Design and Objectives of the Phase 2a Study of Aerosurf
The phase 2a study was designed to investigate safety and tolerability of Aerosurf in neonates who were between 29 and 34 weeks gestational age. The primary population for Aerosurf is babies between 24 and 36 weeks but because this was the first study in babies, it was judged appropriate to begin in somewhat older babies who have fewer complications of pre-maturity and more physiological reserve. After gaining information in these older babies, it then is appropriate to move to treating more fragile babies in subsequent studies. The neonates in the study at the time of enrollment were receiving nCPAP for RDS. They had to be enrolled within 21 hours after birth and to have received nCPAP within one hour of birth. They had to be able to breathe spontaneously and to have radiographs consistent with RDS.
This was a randomized, controlled trial conducted at 4 medical centers in the US. There were three different cohorts who were assigned different doses; each cohort received just one dose. Each cohort was comprised of 8 babies on Aerosurf added to nCPAP and a control group of 8 babies on nCPAP. Hence, the overall study treated 24 babies with Aerosurf added to nCPAP and 24 with nCPAP alone.
The aerosolized KL-4 was introduced into the nCPAP circuit. The doses contained the same concentration of KL-4 but were delivered over 15, 30 and 45 minutes which corresponded to doses of 25, 50 and 75 mg/ kg. Just to emphasize this point, the same concentration of aerosolized KL-4 surfactant was given to every baby, but the dosage was increased by lengthening the time of administration.
There were three objectives for this trial. The primary objective was to establish safety and tolerability of Aerosurf as compared to nCPAP. The two secondary objectives were to determine that:
- The capillary aerosol generator (CAG) was effective and could be easily incorporated into the work flow in the NICU, and
- Physiological data measurements could indicate that aerosolized KL-4 surfactant was being delivered to the lungs of premature infants and producing effects consistent with surfactant replacement.
Safety Results for Aerosurf
Tolerance to the Medical Device Used to Deliver Aerosurf
The patient interface and nasal prongs were well tolerated. One of the potential risks of surfactant delivery is upper airway obstruction as a result of the nCPAP device, but physicians did not report this as a problem. Only one baby could not be given the prongs due to an anatomic limitation, but it was still able to be dosed.
Peridosing events were infrequent
When surfactants are administered via an intratracheal tube bolus as in mechanical ventilation, dosing needs to be interrupted in 4% to 9% of the patients. Reflux or obstruction of the endotracheal tube occurs in 2% to 18% of babies resulting in desaturation, pallor and bradycardia. In the Aerosurf group of 24 babies there were no cases of bradycardia, 3 (13%) of desaturation and 1 (4%) of vomiting. This was a very acceptable peridosing profile.
Adverse Events Typically Seen in Neonates with RDS
Investigators looked at adverse events other than RDS that are common in neonates; these are neonatal jaundice, constipation and apnea. These are events that are expected for this population. Incidence was in the expected range and occurred equally between Aerosurf and the control group. Importantly, there was no indication of an increase in adverse events with increasing Aerosurf dose.
Serious Adverse Events
In terms of serious adverse events there was 1 case (4.2%) of apnea 1 case (4.2%) of necrotizing enterocolitis in the Aerosurf group versus none in the control group. There was 1 case (4.2%) of cardio-respiratory arrest and 1 case (4.2%) of intraventricular hemorrhage in the control group versus none in the Aerosurf group. Hence serious adverse events had the same incidence in each group.
Pneumothorax/ pneumomediastinum Occurrence
Last year, management told investors that in an interim look there were numerically more cases of pneumothorax/ pneumomediastinum (air leaks from the lungs) in Aerosurf babies than control nCPAP babies. This information was based on results from the first two of three cohorts being dosed. These air leaks can occur because of high volumes of gas and pressure in the lungs leading to a tear in the lungs. The air leaks proved not to be an issue when looking at the entire population. There were 4 cases (16.7%) of pneumothorax/ pneumomediastinum in the control group and 5 (20.8%) in the Aerosurf group. Again there was no incidence of increasing adverse events with increasing dose in the Aerosurf group.
In looking at all air leak events, there were 5 cases (20.8%) of pneumothorax/ pneumomediastinum in the control group and 20.8% in the Aerosurf group. There was 1 case of pneumothorax in the control group that was reported as non-serious, There was 1 case (4.8%) of pulmonary interstitial emphysema in the control group and none in the Aerosurf group. These results for Aerosurf and the control group are consistent with the incidence of air leakage reported in medical literature of 23% to 47%.
Management’s Assessment of Safety and Tolerance
Management concluded that the adverse events seen in this trial were consistent with those that would be expected in this population. The incidence of adverse events and serious adverse events were comparable between the Aerosurf and control groups. There was no pattern of increased adverse events with increasing Aerosurf dosing. The capillary aerosol generator delivered KL-4 in a safe and well tolerated way. The independent Safety Review Committee concurred and approved moving to the next study.
Performance of the Capillary Aerosol Generator (CAG)
Another key objective of the trial was to assess the performance of the capillary aerosol generator in the NICU setting. The collaboration with Battelle brought world class medical device technology and engineering expertise to the clinical development of this device. Interestingly, this technology was created at considerable cost by Phillip Morris which was looking at safer ways to deliver nicotine to the lungs. Discovery licensed the technology for this indication in December 2005.
This trial was the first human application of the capillary aerosol generator technology. Management’s assessment was that the CAG performed as designed. It delivered a high output dense aerosol stream that met all design criteria. There were no device failures during treatment and there were no device related adverse events. The device was well accepted at all NICU sites. Aerosurf appears to be safe and the aerosol generator seems dependable and user friendly. There have been several attempts in the past to aerosolize animal surfactants using off the shelf nebulizers, which ended in failure. They tended to clog up and couldn’t deliver consistent flow.
Did the KL-4 Surfactant Perform as Expected?
Recognizing that the numbers of patients treated was a small sample and only one dose was given, any results can only be taken as indicative. Of course, what investors are extremely interested in is whether there might be any physiological evidence that KL-4 surfactant is being delivered in efficacious amounts that meaningfully improved lung function. There were encouraging signals, but because of the small size of the trial, these do not constitute proof of concept.
Investigators were hoping that fewer babies given Aerosurf would require a change to mechanical ventilation relative to the control group. They looked at time to intubation and rates at which babies needed to move to mechanical ventilation in both groups. It was also important to look at the requirement for more oxygen (FiO2) levels of CO2 partial pressure in the blood.
Effect on Gas Parameters
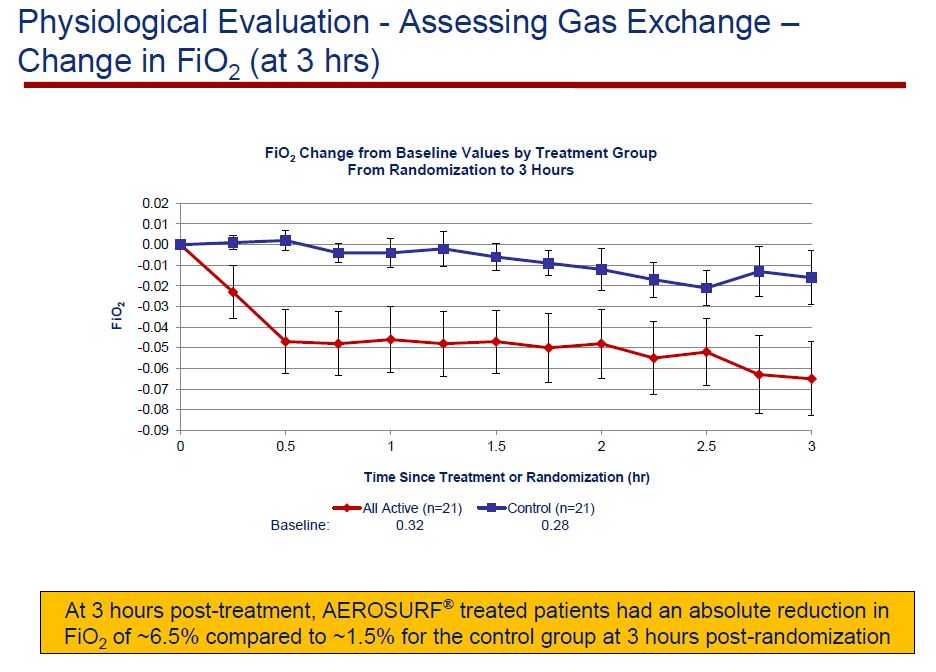
Increasing levels of FiO2 can cause toxic effects and there is a direct relationship of failure of nCPAP to increased FiO2 levels. Consequently, an important goal of Aerosurf therapy is to decrease FiO2 or hopefully return it to a normal level of 0.21.The Aerosurf group had a baseline FiO2 of 0.32 and the control group had 0.28. Evaluations were made in 22 patients on Aerosurf and 22 on control. Over a three hour period, a consistent improvement of FiO2 in favor of the Aerosurf babies was seen.
At one hour post Aerosurf treatment, 36% of Aerosurf babies were at a FiO2 level of 0.21, which is room air, and did not need supplemental oxygen; this compared to 14% in the control group. This was compared to a measurement of the control arm for which the measurement was taken one hour post-randomization. At the 3 hour time point the Aerosurf group had an absolute reduction in FiO2 of 6.5% versus 1.5% in the control group.
The other part of assessing gas exchange in the lungs is CO2 pressure measured in the blood. When gas exchange is impaired, CO2 levels can increase; this is a signal that airways are being clogged and air can’t be efficiently exhaled. The initial trial protocol did not call for measurement of CO2 levels in the first dose group. This was amended to take measurements in the second and third cohorts. At 3 hours there was a 9 mm Hg decrease in CO2 versus 1.5 mm Hg in the control group. The magnitude of this effect is clinically relevant. It is also consistent with the FiO2 data suggesting that a favorable surfactant effect of gas exchange in the lungs.
nCPAP Failures Need for Rescue Therapy and Requirement for Invasive Respiratory Support
The goal of Aerosurf is to increase the number of babies that can be treated with nCPAP alone and not require rescue therapy with intubation and mechanical ventilation. The study looked at nCPAP failures and the need for reintubation over 12 hours. No Aerosurf treated babies required reintubation at three hours post dose compared to 17% of controls. At 6 hours post dose, 13% of Aerosurf babies required intubation versus 21% of controls. However, at 12 hours, the rate of failure was the same at 33%. It is not shown in the next table, but this is also true at 72 hours post dosing.
In the control group 14 of 24 (58%) of babies failed nCPAP. This rate is higher than you might expect in babies in this gestational age range. This can’t be immediately explained but is likely due to the inclusion criteria, which many have led to enrollment of a more at risk population. In this study, they could only administer a single dose of KL-4 surfactant. It is highly likely that more than one dose would improve results. In babies intubated and given mechanical ventilation, about 30% may require more than one dose initially and up to 40% may have to be reintubated because of worsening lung disease and given another dose of surfactants. It is reasonable to expect that higher doses and repeat doses will be helpful in reducing the rates of nCAP failure.
Management Summary of Phase 2a Data
Management assessed the phase 2a results babies of 29 to 34 weeks gestational age as follows:
- Safety and tolerability allows them to move to the next stage of evaluation. Side effects are comparable to control nCPAP group. The internal review and safety board overseeing the trial concurred and has recommended that development continue to the next stage trial.
- The novel aerosol technologies involving the medical device –capillary aerosol generator) and lyophilized KL-4 surfactant are functioning as designed.
- Physiological data is suggesting that KL-4 surfactant is getting into the lungs and is having a drug effect. These include FiO2 requirements, CO2 pressure decreases, time to nCAP failure and intubation and effect on NCAP failure at the highest dose.
One of Discovery’s key opinion leader advisers on the trial noted that there several clinical studies in the past 15 years with the same goal as Aerosurf. These studies attempted to aerosolize animal derived surfactants using different off the shelf nebulizers. He noted that none of these had successful results but above all, none of these studies used an aerosol generator and aerosol system that was designed for ventilated infants with RDS.
Next Clinical Development Steps
Discovery management says that members of the Aerosurf Steering Committee, which is composed on neonatology thought leaders, are encouraged by the study and have recommended moving forward to the next stage trials. The Company has engaged a Contract Research Organization with experience in neonatology to conduct these trials. They are adding investigational sites in the US and will also bring on sites in Canada, Latin America and the EU; the phase 2a trial was only conducted in the US. Phase 2a results will be prepared for publication and presented at major medical conferences.
The immediate next step is another phase 2a study. This is needed to better understand the effect of higher doses and repeat doses. It will be exploring higher doses and repeat dosing in babies of 29 to 34 weeks gestational age. It will also enroll younger babies of 26 to 28 weeks gestational age as compared to just reported phase 2a trial which was in older babies of 29 to 34 weeks gestational age. The Company says that this second phase 2a will complete in 4Q, 2015.
They will then begin a phase 2b study will begin in 4Q, 2015 or 1Q, 2016. It will enroll babies of 26 to 32 weeks gestational age. The level and frequency of dosing will be informed by the second phase 2a. It will be done at centers in the US, Canada, Latin America and the EU and is scheduled to complete in mid-2016. This will be the all-important proof of concept trial that will demonstrate the clinical effect and the dose to take forward into the phase 3 program.
Phase 2b may enroll 200 to 250 patients. It will take 24 to 26 weeks to enroll. Assuming it begins in early 2016, it could complete in 3Q, 2016 and issue topline results in 4Q, 2016. FiO2 and CO2 will be monitored continuously. The baseline FiO2 requirement may be dropped from 0.30 to 0.25 in the first phase 2a. Patients will get a second dose if they meet certain criteria. They will need $20 to $25 million to get through phase 2b results.
Given disappointing delays in enrolling the phase 2a trial, investors are concerned about the ability of the Company to meet the timelines it has given for phase 2b. The Company points out that it now has more sites on line that the four sites they had a year ago which were used to start the phase 2a. They had increased this to 10 sites at the end of 2015. More and more sites are coming on board and they plan to have 20 sites in the US and a handful of sites abroad by 4Q, 2015.
Tagged as Aerosurf, Discovery Laboratories Inc, DSCO, nCPAP and Aerosurf, Windtree Therapeutics + Categorized as Company Reports





Very interesting Larry. Subscribers really get their money’s worth with these stories.