Agenus: Encouraging Phase II Results for Prophage in Recurrent Glioblastoma (AGEN, $3.00)
Purpose of This Report
Agenus issued a press release on December 16, 2013 in regard to the final results of a phase II trial of Prophage G-200 in recurrent glioblastoma based on an article in the journal Neuro-Oncology, the official journal of the Society of Neuro-Oncology. This was an open-label, single-arm, phase II study. Patients enrolled were adult patients with surgically resectable recurrent glioblastoma. They were given Prophage after gross total resection.
The primary objective of this trial was to assess the survival rate at six months post-surgery. Secondary endpoints were progression-free survival, safety, and immune profiling. This report discusses results and tries to put them in perspective.
Key Points of This Report
The data suggests that Prophage performs as well or better than the data from a phase II trial that led to accelerated approval of Avastin in recurrent glioblastoma. Obviously, comparing data from two separate trials cannot be viewed with as much confidence as data from a randomized trial. Nevertheless, the data can only be seen as encouraging.
As a result of this trial, a new, 222 patient phase II trial comparing the combination of Avastin and Prophage to Avastin alone is now underway with topline results possible in 2015. This new study is called the "ALLIANCE" trial. The National Cancer Institute is funding the ALLIANCE trial at an estimated cost of over $20 million in industry dollars; Agenus is only manufacturing the vaccine for the trial. If successful, the ALLIANCE trial could be sufficient basis for approval of Prophage in recurrent glioblastoma based on the FDA's action in approving Avastin.
Prophage Phase II Results
Prophage is an individualized cancer vaccine that is manufactured using tissue obtained from a patient's own tumor during surgery. Each vaccine contains the 'antigenic fingerprint' of the patient's particular cancer and is designed to activate the patient's immune system to specifically target and destroy cancer cells bearing this fingerprint.
The Phase II trial enrolled 41 patients with a mean age of 55 years with surgically resectable, recurrent glioblastoma. Patients enrolled had a gross total resection in which ≥90% of their tumors were removed.
Patients were treated after surgery with Prophage once weekly for four weeks, followed by biweekly injections until vaccine depletion. The primary objective of the trial was patient survival at six months.
Between October 3, 2007 and October 24, 2011, 41 patients underwent gross total resection of recurrent glioblastoma and received a median of 6 doses of Prophage. Following treatment, 90.2% of patients were alive at 6 months (95% confidence interval [CI]: 75.9-96.8) and 29.3% were alive at 12 months (95% CI: 16.6-45.7). Median overall survival was 42.6 weeks (95% CI: 34.7-50.5).
There were no serious adverse events associated with vaccine administration. There were 37 serious (grades 3-5) adverse events reported, with 17 attributable to surgical resection and only a single grade 3 event related to Prophage. As seems to be the case with most cancer vaccines, Prophage has a benign side effect profile.
I think that it is important to point out that Agenus was not a direct participant in this trial and did not fund the trial. This was an investigator led study by Dr. Andrew Parsa. He is an MD surgeon with a PhD in immunology who is currently Chair of the Department of Neurological Surgery at the Northwestern University Feinberg School of Medicine.
Dr. Parsa did not receive any financial support or travel expense reimbursement for his work or for consulting activities on behalf of Agenus. Dr. Parsa does not have an equity interest in Agenus or a financial relationship with the company. The trial was funded by the American Brain Tumor Association, Accelerated Brain Cancer Cure, National Brain Tumor Society, and National Cancer Institute Special Programs of Research Excellence. Agenus' only role in the trial was to manufacture the product.
How Good Are These Results
I compared these results to those of a phase II trial of 167 patients who were given either Avastin alone or Avastin combined with irinotecan that was a significant basis for approval of Avastin in recurrent glioblastoma. These results were published in the Journal of Clinical Oncology: Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733-4740. I have compared the Prophage results with some of those in the Friedman study in the following table.
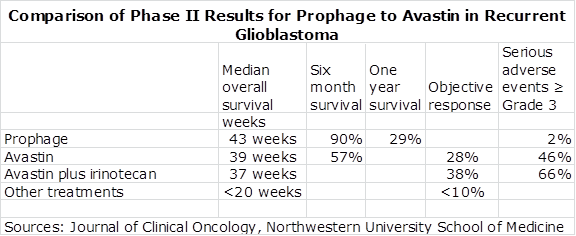
It can be seen that the results for Prophage were slightly better than Avastin alone and Avastin plus irinotecan in terms of median overall survival and that Prophage seemed meaningfully better in terms of six month survival. Also, Prophage appears to be a much safer drug with only 2% of patients experiencing a grade 3 or worse side effect as compared to 46% for Avastin.
These results must be looked at in the context that the data for Prophage and Avastin came from separate trials. We would need a randomized trial of Prophage against Avastin to determine if these results are real. This is the purpose of the ALLIANCE trial that is now underway. That said, this data in my opinion, does indicate that Prophage acts as well as Avastin in this setting with a much safer side effect profile. This phase II data was the basis for an accelerated approval for Avastin in recurrent glioblastoma.
What Comes Next, ALLIANCE
A new phase II trial called ALLIANCE is now underway; it is a randomized phase II trial. This is a three-arm study that will enroll approximately 222 patients with surgically resectable recurrent glioblastoma. One arm of the trial will be Prophage and Avastin; the second arm will be Prophage plus Avastin in patients who have progressed after Avastin; and the third arm will be just Avastin. This study design reflects the view that Prophage and Avastin will be synergistic even in patients who have failed Avastin. The primary endpoint is overall survival.
This study is being sponsored by the Alliance for Clinical Trials in Oncology (ALLIANCE), a cooperative group of the NCI. The NCI is funding the costs of the trial which based on an estimated cost per patient of $100,000 could cost $22 million. Agenus is not providing any of the funding, but will manufacture the product. The NCI made the decision to fund this study based on the phase II interim results. The final study results were published today in Neuro-Oncology.
Are There Any Implications for Other Cancer Vaccines
The mechanism of action for Prophage is based on its heat shock protein technology that is beleived to result in the loading of dendritic cells with all cancer antigens expressed in the tumor. Northwest Biotherapeutics has the same objective with its technology that has resulted in DCVax-L. ImmunoCellular's ICT-107 results in only six antigens being presented by dendritic cells regardless of whether they occur in the tumor. Does this phase II result mean that AGEN and NWBO have a greater chance of achieving efficacy than ICT-107? I don't know.
Note to Adam Feuerstein
I have had a keen extensive interest in cancer vaccines aimed at glioblastoma that are in development by Agenus, Celldex, and Northwest Biotherapeutics. Feuerstein has consistently attacked me when I have published on these companies. Without really stating any substantive reasons, he believes that Agenus and Northwest Biotherapeutics will fail. I don't know if he has an opinion on Celldex.
While he has not seen fit to meaningfully challenge my analysis of these companies, he has launched a series of personal attacks alleging that I am pumping and promoting penny stocks to retail investors. He claims that I am guaranteeing that the trial of DCVax-L and Prophage will work and I can only respond that I do not know if the trials will work. However, there is a reasonable basis for conducting a trial and in my opinion the risk adjusted potential for success significantly offsets the potential for losses in the event of trial failure.
Dr. Parsa and the NCI believe that the data supporting Prophage is sufficiently convincing as to warrant a large-scale, phase II randomized trial that could be registrational. By Feurstein's logic or whatever he relies on in writing his articles Dr. Parsa and the NCI are joining with me in pumping and promoting penny stocks to retail investors. You be the judge.
Tagged as Agenus + Categorized as Company Reports




