Northwest Biotherapeutics: Issues to Focus on in Pending Manuscript Dealing with Blinded Data from Phase 3 Trial of DCVax-L in Newly Diagnosed Glioblastoma (NWBO, Buy, $0.23)
Purpose and Summary of This Report
My key takeaway points are summarized at the end of this report. I urge you to first go through the full text, but if for whatever reason you are impatient, you can start from the back of the report.
The primary purpose of this report is to provide data against which to judge results from an important pending manuscript on the phase 3 trial which I anticipate will be published in a peer reviewed medical journal in the near future. This is being prepared by the investigators participating in the trial. The Company first told investors that this manuscript was in process in a presentation at ASCO on June 5, 2017 and reiterated this guidance in 2H, 2017. It has given no recent guidance on the timing of publication, but I am guessing that it will not be that much longer.
The Phase 3 trial remains blinded. However, key investigators in the trial have stated publicly that patients appear to be living meaningfully longer than would be expected if they had been treated only with standard of care (SOC). The Company has indicated that these investigators are preparing a paper that will discuss the blinded data. Wait a minute! Isn’t it usually the case that you can’t get much of an insight, if any, from blinded results? The answer is almost always yes to this question, but this trial appears somewhat unique.
Let me reiterate that the purpose of this note is to give investors benchmarks against which to judge results as presented in the pending manuscript. I am making no projections potential results in this note, but there are reasons to believe that they will be encouraging.
- Phase 1/2 results in 20 patients showed a striking median overall survival (mOS) of 36 months.
- Data from the information arm of the phase 3 trial were encouraging. See this link.
- A presentation by the Company at ASCO in June 2017 about unblinded data offered further encouragement See this link.
- The lead investigator and discoverer of DCVax-L is the distinguished neurosurgeion Linda Liau. She is (1) Chair, Department of Neurosurgery (2) Professor, Department of Neurosurgery, and (3) Director, Brain Tumor Program, at the UCLA School of Medicine. While she has not seen unblinded data, she has said that patients appear to be living longer in the phase 3 trial. She has no economic interest in NWBO.
Hopefully we can glean information on how median overall survival (mOS) and other data on survival for all 331 patients compares to historical results for SOC. While the trial remains blinded, investigators will know mOS for the 331 patients and also how long each patient has survived although they won’t know what treatment the patient received. However, because of the cross over design of the trial we know this:
- The 331 patients who were enrolled in the trial were randomized 2:1 so that I estimate that about 221 were initially given DCVax-L plus standard of care
- Approximately one hundred and ten (110) patients were started on SOC but according to the trial protocol, DCVax-L was then added to SOC for approximately 76 patients when their cancer progressed
- This means that only roughly 34 patients received just SOC
- This trial is essentially a one arm study of DCVax-L as about 90% of patients (roughly 297 out of 331) received DCVax-L
This trial started in 2007 and median enrollment occurred in May 2014. Historical results gleaned from other controlled trials indicate that for glioblastoma patients given SOC, mOS is about 16.5 months (50% have died), 70% die at two years and 85% die by year three, 92% by year four and 95% by year five. Given the unusually long time the trial has been underway, we should be able to compare results for the blinded group against historical SOC results at one, two, three, four and five years. The last patient enrolled in the trial over two years ago so that the chance of that last enrollee being alive at two years if treated with SOC would be 30%. For all other patients, the chance of being alive at this point in time would be much (much) less assuming they were on SOC.
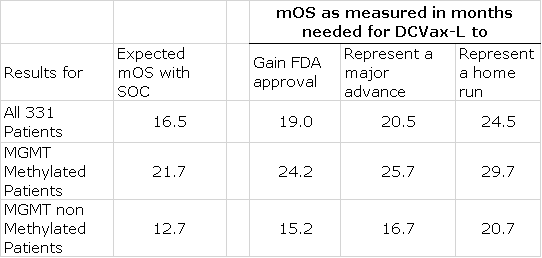
I have estimated the regulatory and medical impact for possible outcomes for mOS in this trial as compared to historical mOS. Based on looking at results seen in other controlled clinical trials in glioblastoma, I estimate that mOS for SOC is about 16.5 months. Because about 90% of the patients received DCVax-L, I am assuming that even though the results are blinded, they are a strong harbinger of results for DCVax-L when the trial is ultimately unblinded. Please reread this paragraph to make sure that you understand this assumption.
- An improvement of 2.5 months or more in mOS for the entire patient population (331 patients; about 90% of whom received DCVax-L)) versus historical SOC (19.0 months versus 16.5 months for SOC) would be quite positive. If these results were later duplicated upon unblinding of the trial, it would very likely result in regulatory approval. The chemotherapy drug temozolomide (last drug approved for glioblastoma) was approved in 2005 on the basis of improving mOS over SOC by 2.5 months.
- Key opinion leaders consider a 4.0 month improvement in mOS for a drug treating aggressive cancers like glioblastoma to be a major medical advance
- An 8.0 month improvement for DCVax-L would be a home run
- Even in the first scenario of a 2.5 month improvement in mOS, I would expect widespread adoption by the medical community assuming that this led to FDA approval.
The key aspect of immunotherapy (like DCVax-L) as has been seen with the checkpoint inhibitors such as Opdivo, is that a minority of patients experience a long survival (the tail). We have seen with Opdivo that in metastatic non- small cell lung cancer (a cancer roughly as aggressive as newly diagnosed glioblastoma) that roughly 15% to 20% of patients are alive at three years versus 5% to 10% for chemotherapy. This means that roughly 10 out of 100 patients treated with Opdivo experience greater three year survival than with chemotherapy in this particular cancer. See my report for more discussion on Opdivo’s survival tail.
This suggests to me that if 25% of patients treated with DCVax-L are alive at three years that it would show a survival benefit similar to that seen with Opdivo, i.e. 10 more patients out of every 100 treated would be alive than if treated with SOC. This long survival tail is what has excited oncologists and has been instrumental in Opdivo and other checkpoint inhibitors reaching $9 billion of sales since being introduced in late 2014. Key opinion leaders will be scrutinizing the data to see if DCVax-L also has a long survival tail.
Here are some other things to consider:
- This long running trial should afford an unusually good insight into the actual survival benefit of DCVax-L. Remember that 85% of patients given SOC die by year 3. The very last patient was enrolled two years ago so that his chances of survival on SOC would be 30%. For regulatory agencies, survival is the gold standard for cancer drug approval and this blinded data and the ultimate release of unblinded data should give a crystal clear understanding of whether DCVax-L increases the length of survival for glioblastoma patients as compared with historical results seen with SOC.
- The last drug approved for glioblastoma was the chemotherapeutic drug temozolomide in 2005. Since then there have been numerous drug failures. Key opinion leaders attribute this striking lack of success to numerous mutations that lead to great heterogeneity in glioblastomas. Other cancer drugs only address one or a small number of these mutations. The mechanism of action of DCVax-L allows a personalized approach to potentially treating the mutation(s) of each glioblastoma patient.
- It is also important to understand the safety of DCVax-L in comparison to life threatening side effects of chemotherapy and other drugs. One of the strong attributes of DCVax-L is its safety profile. The most frequent side effects are a modest fever after injection that can be treated with Tylenol, and mild pain and irritation at the site of intradermal injection. Out of more than 2,000 intradermal injections given to over 400 patients, there have only been 7 patients who experienced serious adverse events that investigators deemed to be related or possibly related to the DCVax-L treatment. Five of these were seizures and it is important to note that because GBM is growing rapidly in the brain that the disease itself can cause seizures. Because safety can be as important to a drug as its efficacy, this is a major plus for DCVax-L.
- Approximately half of glioblastoma patients have the promoter region of the MGMT gene methylated. In methylated MGMT, the expected mOS for SOC is 21.7 months and for non-methylated it is 12.7. In effect, these are two different populations that may need to be considered separately. I am guessing that data on these two interesting sub-groups will be provided. In a later section of this report I include an extensive discussion of the effect on survival of a methylated promoter region of the MGMT gene for those who want to dig deeper.
Key Survival Data to Watch for in the Manuscript
I think that this blinded data will show when a patient enrolled in the trial, if they are still alive or if they died. Expressed another way, it should show the length of survival for each patient in the trial. However, it won’t show if the patient was on DCVax-L plus SOC or just SOC. The clinical trial for DCVax-L was begun over 10 years ago, median enrollment was reached in May 2014 (3 ½ years ago) and the final patient was enrolled in November 2015 (2 years ago). The average expected survival (50% still alive) for a newly diagnosed GBM patient is about 16.5 months or 1 1/3 years. I estimate that 70% are dead at two years, 85% at three, 92% at four and 95% at five. We should be able to gain a really good insight into whether patients in the trial lived meaningfully longer than would be expected with SOC.
Investigators in the trial can compare survival results for each patient against the expectation that the average patient will die at 16.5 months. Importantly, the trial protocol included a cross over design in which patients started on SOC were switched to DCVax-L if their cancer progressed. Because of this feature, about 90% of patients in the trial received DCVax-L. The importance of this is that in looking at blinded survival outcomes, investigators can assume that improvement in survival in the trial, if any, is largely determined by the therapeutic effect of the drug. While we won’t know the actual results for DCVax-L until the trial is unblinded sometime in the future, the blinded survival results are a very strong proxy of what the final trial results will show.
It will be extremely important to look at results not only for all 331 patients enrolled in the phase 3 trial of DCVax-L, but also at the status of MGMT methylation (I go into detail on this later in the report). Patients with MGMT methylation have better survival prospects. Their median overall survival expectation, according to the literature search I have done is 21.7 months versus 12.7 months for patients without MGMT methylation. My work further suggests that about half of patients with GBM have MGMT methylation. Because of the great difference in survival prospects, it will be important to look at the status of MGMT methylation in each patient and providing this data can be done without unblinding the trial. However, I am not sure if the pending release will have data based on MGMT methylation status. There may also be data on other important sub-groups.
In interpreting the pending data release, I am almost totally focused on length of survival for each patient and also mOS for all 331 patients in the trial. Remember that I am assuming that mOS results for this still blinded trial is a strong proxy for DCVax-L results when the trial is eventually unblinded and topline results released. I am also comparing these results to historical mOS outcomes in other trials. The next issue to address is how to interpret improvements, if any, in mOS. Here are some key data points:
- The Stupp trial established temozolomide plus radiation as the current SOC in newly diagnosed GBM replacing surgery. In that trial, the combination of temozolomide and radiation improved mOS by 2.5 months over radiation alone. This led to regulatory approval and nearly universal usage in clinical practice. Based on this, I assume that if DCVax-L can show a 2.5 month increase in mOS over SOC that like temozolomide, it will likely gain regulatory approval and widespread usage.
- The rule of thumb in oncology is that a 4.0 month improvement in mOS for an aggressive cancer like GBM is a major medical advance. The medical device Optune was recently approved on the basis of a 4.0 month improvement.
- An improvement of say 8.0 months in mOS would be extraordinary.
Here is a summary of how improvements in mOS that will soon be reported in the manuscript could be interpreted.
Further Perspective on the Trial
Although these numbers give the appearance of great precision, this may not be the case. I acknowledge this, but I believe that the mOS results from the unblinded data will afford extremely valuable insights into determining if DCVax-L has a therapeutic effect and how powerful it is.
Enrollment Insights
The trial enrolled 331 patients and was randomized 2:1 in favor of DCVax-L so that roughly 67% or 221 of the 331 patients enrolled in trial initially should have received DCVax-L plus standard of care (SOC) and approximately 110 initially received only SOC. The trial protocol allowed for patients whose cancers progressed on SOC to be switched to DCVax-L. Very importantly, patients who progressed on DCVax-L continued to remain on DCVax-L because trials with other immune therapies like the checkpoint inhibitors have shown survival benefits even after the cancer has progressed. This was all done in a blinded fashion.
The Company commented in a June 5, 2017 presentation at ASCO that about 90% of patients in the trial received DCVax-L at one time. We know that by design approximately 221 patients (67%) were started on DCVax-L. By simple arithmetic, we can calculate that 23% or 76 of the patients first received SOC and were switched to DCVax-L and that 10% or about 34 received only SOC. The importance is that results in the trial give a meaningful insight into the therapeutic effect, if any, of DCVax-L. This is essentially a one-armed trial.
There May Not Be a Meaningful Control Group
I know that some of you are now thinking that because around 297 patients were on DCVax-L and only 34 were on SOC that we may not have a meaningful comparison of DCVax-L to SOC. I think this is largely correct and, if so, regulators will have to compare DCVax-L results to historical results for SOC as demonstrated in other trials. I know that some of the habitual attackers of Northwest will cite this lack of a large control group as a reason why the FDA might reject results from the trial. Let me point out why I think that this will not be the case.
The first thing I would cite is that Gilead’s CAR-T product, Yescarta, was approved for r/r DLBCL on the basis of only 101 patients who received Yescarta. There was no control group. Similarly, Novartis’ CAR-T product Kymriah was approved on the basis of only 61 patients with pediatric r/r ALL treated with Kymriah. In each case, there was no control group and the FDA agreed to compare these results to historical SOC results. We have also seen the FDA accept historical results in some approvals of the checkpoint inhibitors Opdivo and Keytruda in certain aggressive cancers. Based on this, I don’t think that comparing DCVax-L results to historical SOC results will be a stumbling block for approval.
Just What Are Historical SOC Results
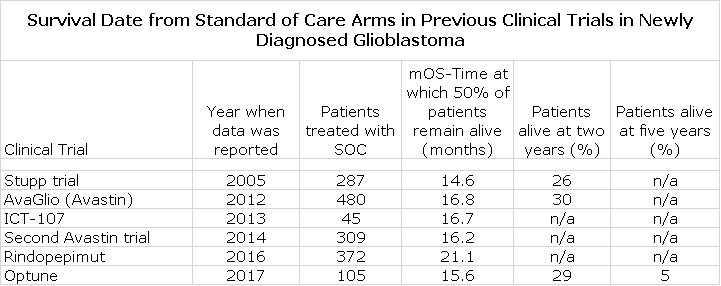
The following table shows results for the SOC arm in six recent, randomized, clinical trials in newly diagnosed glioblastoma.
I want to call your attention first to the results from the Stupp trial. In this trial, patients were enrolled even if their cancer had progressed before starting treatment. In all other trials, and in the DCVax-L phase 3 trial, these rapid progressor were excluded. This probably explains the lesser mOS in the Stupp trial. In the rindopepimut trial, only patients with the EGFRvIII mutation were included. Patients with this mutation comprise about 30% of the newly diagnosed GBM population. Because of this, I think that the 21.1 months of mOS seen in the control arm is applicable to this sub-group and not the broad population. You can see that the other trials show a tight range of 15.6 to 16.7 months for mOS. In this report, I estimate that the expected mOS for SOC is 16.5 months.
Only the Optune trial provided data beyond two years and indicated that 16% of SOC patients were alive at three years post-surgery, 8% at four years and 5% at five years. I next did a literature search to try to find data showing survival beyond two years. I found a meaningful number of studies which were not randomized and as rigorously conducted as the six studies just cited. One of the better summaries that I looked at can be found at this link. Based on this additional work, I filled in the gaps with estimates of survival at three, four and five years as shown below:
Manuscript Will Afford Extremely Important Data on Survival
There is another factor working in favor of DCVax-L when regulators view results from this trial and that is that there will be extensive information on survival which is the gold standard endpoint for any oncology trial. The median enrollment in the trial occurred in May 2014 (over 3 1/2years ago) meaning that half of the patients enrolled before then and half after. The last patient enrolled in November 2015. This is important because we know that about half of patients with newly diagnosed GBM die within 16.5 months or so. Hence, we should be able to get an excellent read on whether DCVax-L is extending life. The last patient enrolled in November 2015 in the trial has only a 30% chance of being alive and a patient enrolled at the median time for enrollment in May 2014 has only a about an 11% chance of being alive.
In the case of Kymriah and Yescarta, the endpoint of the trial was the degree of shrinkage of the tumor, a much less solid end point than survival. There was no data on survival. Hence, the FDA will have much better information on survival than it had when it approved Kymriah for pediatric r/r ALL and Yescarta for r/r DLBCL. Newly diagnosed glioblastoma is only slightly less aggressive than r/r ALL and r/r DLBCL in terms of expected survival.
Let me hammer on a key point. This trial is likely very unique in the amount of information it will provide about the length of survival for the trial as a whole and also for sub-groups of patients. The data set will give much more definitive data than the long term survival effects of the CAR-T drugs Kymriah and Yescarta. Indeed, the DCVax-L trial is an outlier among cancer trials in terms of the quality of information that it will afford on survival.
Perspective on the Importance of Methylation of the MGMT Promoter Region
The status of methylation or non-methylation of the promoter region of the MGMT gene will be something of keen interest as MGMT methylation results in much longer survival. Accordingly, I have done some significant analysis on this. This section is pretty technical and is not essential to understanding the points that I make in this note.
Understanding MGMT Promoter Methylation
In interpreting the results of the phase 3 trial of DCVax-L in newly diagnosed glioblastoma multiforme, it is important to understand the role of the MGMT gene in patient outcomes. The MGMT gene codes for a protein (O6-methylguanine–DNA methyltransferase) that repairs damage to DNA. This is important because its activation negatively affects the activity of the chemotherapy drug temozolomide. Standard of care (SOC) for newly diagnosed GBM starts with surgical resection followed by radiation and temozolomide. Temozolomide belongs to a class of chemotherapy drugs called alkylating agents that damage DNA and impairs replication; this leads to cancer cell damage or death.
High levels of the protein coded by MGMT can repair the DNA damage done to cancer cells by temozolomide and worsen patient outcomes. The transcription of the MGMT gene by RNA is initiated by a segment of DNA that is called the promoter region. This promoter region for some GBM patients is methylated. When this occurs this silences (blocks) the transcription of the protein coded by MGMT and does not undo the DNA damage caused to cancer cells by temozolomide. This is referred to as MGMT promoter methylation or more simply MGMT methylation. Patients with MGMT methylation have a better survival outcome. This is a bit confusing, but it can be thought of as follows:
- MGMT methylation is good for survival: The MGMT DNA repair mechanism that negatively interferes with the effect of temozolomide is blunted and patient outcomes are improved.
- MGMT non-methylation is bad: The MGMT DNA repair mechanism is not blunted and patient outcomes are worse than that for patients with MGMT methylation.
Effect of MGMT Methylation in Outcomes for Newly Diagnosed GBM Patients
In my literature search, one of the best articles I found appeared in the New England Journal of Medicine. It was MGMT Gene Silencing and Benefit from Temozolomide in Glioblastoma. This was a small study that looked at 507 newly diagnosed GBM patients treated with either radiation alone or radiation followed by temozolomide. Methylation status test were performed on 307 patients and the methylation status of 206 patients was evaluable. Of the 206 evaluated tumors, 92 (44.7%) had detectable MGMT methylation whereas 114 (55.3%) did not. The proportion of methylated tumors was similar in the two treatment groups; i.e. radiation or radiation plus temozolomide. In both treatment groups MGMT methylation improved outcomes. Bear in mind that radiation, like temozolomide, also disrupts DNA so that increased levels of the MGMT coded protein could reduce the effect of radiation as well.
Outcomes According to MGMT Methylation Status
With MGMT Methylation
Here are the results for patients with MGMT methylation. The median overall survival was 21.7 months for patients receiving radiation plus temozolomide as compared to 15.3 for radiation alone.
- The two year survival rate for patients receiving radiation plus temozolomide was 46.0% versus 22.7% for radiation alone.
- Median progression-free survival was 10.3 months for patients with temozolomide plus radiation as compared with 5.9 months for patients who received radiotherapy alone
Non-Methylated MGMT
For non-methylated MGMT patients,
- The median overall survival for patients treated with temozolomide and radiation was 12.7 months versus 11.8 months for radiation alone.
- The two year survival was 13.8% with temozolomide plus radiation and less than 2.0% for radiation alone.
- Median progression-free survival of 5.3 months for patients with temozolomide plus radiation as compared with 4.4 months for patients who received radiotherapy alone
Combining Results for MGMT Methylation and Non-Methylation for Both Treatment Groups
For the entire estimated population of 206 patients for whom MGMT status could be evaluated, there was a significant difference, irrespective of treatment assignment, in overall survival between patients whose tumors had MGMT promoter methylation and those whose tumors did not (P<0.001). The hazard ratio for death was 0.45 (95% confidence interval, 0.32 to 0.61) among those with MGMT promoter methylation, a result that corresponds to a 55 percent decrease in the risk of death in this subgroup. The median overall survival among patients with promoter methylation was 18.2 months (95% confidence interval, 15.5 to 22.0), as compared with 12.2 months (95% confidence interval, 11.4 to 13.5) among those without methylation.
Problems with This Data
This was a small study not powered for significance, which reduces the reliability of the data. It was not randomized for risk factors other than methylation status. In addition, the results are somewhat confounded because of patients in the temozolomide plus radiation group, 58% received salvage chemotherapy (some other alkylating agent) and 25% were retreated with temozolomide. In the radiation group, 70% received salvage chemotherapy and 60% received temozolomide. This subsequent or salvage therapy may have improved outcomes in both treatment groups.
Key Takeaway Points
Here are the key takeaway points:
- I am expecting a manuscript discussing blinded data from the 331 patient phase 3 trial of DCVax-L in newly diagnosed glioblastoma will be published in a peer reviewed journal in the immediate future. This paper has been co-authored by most of the investigators in the trial.
- The design of the trial allowed for patients started on SOC to receive DCVax-L if their cancer progressed. As a result, I estimate that: (1) about 221 patients were started on DCVax-L, (2) about 110 patients were started on SOC and roughly 76 of these patients were given DCVax-L when their cancer progressed, and (3) only around 34 patients received just SOC.
- This trial is essentially a one armed study of DCVax-L. The results for mOS as shown in the expected manuscript should be an excellent proxy for what mOS will be when the phase 3 results are unblinded at some future point.
- There is great deal of historical data that shows that mOS for SOC is about 16.5 months. As discussed in this paper, if the mOS for the blinded 331 patients shows a 2.5 month improvement in mOS (19.0 months versus 16.5 months), there is a strong chance for approval. The last drug approved in newly diagnosed glioblastoma was temozolomide in 2005. It showed a 2.5 month improvement over what was then SOC. Subsequently temozolomide was included as part of SOC and given to almost all GBM patients
- Key opinion leaders consider an improvement of 4.0 month in mOS in an aggressive cancer like newly diagnosed GBM to be a major advance.
- An 8.0 month or longer improvement in mOS would be a home run.
- This data should also give an idea as to whether there is a survival tail. If roughly 25% of patients are alive at three years in this trial, this would compare to 15% expected with SOC. In other words 10 more out of 100 treated with DCVax-L would be alive at three years than would be the case if they received just SOC.
- Why is that important? It is because the checkpoint inhibitors like Opdivo and Keytruda had survival tails like this in metastatic non-small cell lung cancer and metastatic melanoma which are aggressive tumors like newly diagnosed glioblastoma.
- It is the survival tail of Opdivo and Keytruda (keeping 10 more patients alive at three years) that excited key opinion leaders and has led to these and other checkpoint inhibitors as a group reaching $9 billion of sales following their introduction in late 2014.
- The mechanism of action of DCVax-L is such that it is reasonable to think that it might show efficacy in most (all) solid tumors-lung, breast, colon, etc. Newly diagnosed glioblastoma might only be the first indication.
- A savage social media attack on the Company combined with extremely aggressive naked shorting has resulted in the stock selling at $0.25 per share. It is priced in the expectation that the trial will almost certainly fail. If DCVax-L is eventually approved as seems possible, this might well be the most amazing story ever told in biotechnology. Let’s hope so.
Tagged as Northwest Biotherapeutics Inc., NWBO, Phase 3 trial of DCVax-L in newly diagnosed glioblastoma + Categorized as Company Reports, LinkedIn






Why such a long gap in date of trial start (2007) and median enrollment (2014)?
During the financial crisis, the equity market was closed and they had to put the trial on hold until they could raise some cash.
Larry, my “objective” concern with the study is that the control group is now so small that there is a heightened risk that overall survival for the non- DCVax-L could randomly be higher than in the other studies. The fact that blinded overall survival data seems to be high, while overall a great thing, also implies that the placebo patients have experienced higher overall survival.
You seem to be assuming that the FDA will use the historical overall survival data as the baseline rather than the data from this study.
I’ve become a cynic about the FDA. Yes, the DCVax-L performance will likely be similarly effective to Yescarta and Kymriah with the added bonus of mild vs. severe, even fatal side effects. Additionally, the DCVax-L data will be far more robust with overall survival data not just progression free data. Nevertheless, Kite (before announcing its results) had a market cap at least 20-30 times higher than NWBO.
It sure seems to indicate that there are known “winners” and “losers” in drug development and that they are identified long before the studies are completed.
I own the stock but I feel that I’m just tilting against windmills.
The small control group will be an issue. However the CAR-T drugs were approved on the basis of much smaller trials (101 patients and 61) with endpoints of objective response rates and without a control group. The FDA relied on historical control data. The DCVax-L trial will have meaningful survival data on every patient. This makes for a much higher quality data set. The extent of survival data is virtually without precedent in oncology clinical trials. Finally, the mOS for standard of care in newly diagnosed glioblastoma is well defined from other trials at around 16.5 months. Remember that GBM is a rapidly growing cancer in the confined interior of the skull. It is an extremely aggressive cancer. In the face of impressive survival data as compared to historical survival, it would be very hard for the agency to insist on doing another study with a control group, but this is the FDA and their behavior can be erratic. Of course, at this point we are just hoping that the data will be good. Perhaps, we are all overly hopeful.
Larry can you comment on the above charts for MOS and % survival. Specifically looking for the difference from when the clock was started and if this was accounted for. Stupp, I believe was from surgery as were both Avastin trials. ICT and DCVAX are from randomization (roughly 3 months after surgery) and I believe Optune was 2 months after surgery. I am not sure on Rindo. Did the numbers above account for the different start times? Along the same lines, did the #s above account for the the differences in start times for % alive at 12, 24, 36. I just want to make sure we are comparing apples to apples.
Standard of care begins with surgical resection that is followed by a regimen of radiation and then chemotherapy that begins a few weeks later. The starting time for measuring survival and progression free survival in all of the GBM trials begins at the time of surgical resection.
Larry, your article is heavily focus on median OS between SOC vs DCVAX L single arm trial. As you pointed out it’s been almost 26 months since the last person enrolled into the trial. How much longer would the company need to wait until they see that tail survival that you said key opinion leaders are looking for? Accoring to some on NWBO that the dilution of shares with warrants have reach the billion mark. Making it harder to see the risk and reward of your buy recommendation. Does this trial need to go on another year to allow everyone a least 3 years of being in the trial before data collecting?
I can only guess and I want to emphasize and re-emphasize that it is a guess that it is in the next few months.
To add to all this, looking at the enrollment curve, and utilizing the expected length of survival table Larry lists above, and applying that to today date, as well as July 1 of 2017 when ~99 patients were still alive, we can estimate what the SOC survival rates should be:
On July 1, 2017, and using a conservative 5% for all patients treated 60 months or more (even those back in 2008) gives an SOC of survival of the 331 patients to be: 56 patients still alive. However, we know that actually 99 where alive.
Using todays date, (and same > = 60 months; 5% survival), gives ~41 patients still alive. And that is a conservative estimate for SOC.