Transcept: The Prescription Trend Watch for Intermezzo is On (TSPT, $6.63)
Introduction
Over the last year, I have written seven articles on Transcept (TSPT) and if you would like to have a more in-depth view of my thinking on the product. I would urge you to review those articles either on Seeking Alpha or my
website SmithOnStocks.com. This report focuses primarily on the progress of the launch of its new insomnia drug Intermezzo.
Intermezzo
Intermezzo is a new formulation of zolpidem, the active ingredient in the insomnia drugs Ambien and Ambien CR, which are now off patent. Intermezzo was specifically designed to treat an insomnia condition that is not well addressed by Ambien and Ambien CR. These drugs are intended to be used before patients go to bed to help them get to sleep and then to maintain sleep during the night. They are not approved for middle of the night awakening in which a patient wakes up in the middle of the night and can't get back to sleep. While Ambien and Ambien CR could be used to treat middle of the night awakening, when uses at their historically prescribed doses they can leave patients groggy and functionally impaired the next morning.
Intermezzo is an exquisitely designed formulation for middle of the night awakening and is the only product approved for this indication. It is a sublingual dosage form that contains about 1/3 of the normal Ambien dose for men and 1/6 for women. It is taken sub-lingually and dissolves under the tongue in about two minutes with a minty taste. It contains a bicarbonate-carbonate buffer that changes pH of saliva which then changes zolpidem into its free base form that is rapidly absorbed into the tisues of the mouth. About one quarter of the dose is absorbed in this way and within ten to fifteen minutes, an effective sleep inducing blood level is reached. This action is much more rapid than Ambien or Ambien CR and causes a rapid return to sleep, so much so that the FDA has required that Intermezzo's label must warn patients to take the drug while in bed. The remainder of the dose is swallowed and as it is absorbed more slowly from the GI tract, maintains sleep for about four hours. Clinical trials show that if Intermezzo is taken four hours before awakening it is not likely to cause a morning after effect.
There are differences in the way men and women metabolize Intermezzo. Women require a dose of 1.75 mg while men require 3.5 mg. and as a result Intermezzo is marketed in gender specific doses. In the case of Ambien the historical bedtime dose has been 10 mg of zolpidem and for Ambien CR the normal dose has been 12.5 mg. Recently the FDA warned that the bedtime dose should be cut in half for these products to reduce the occurrence of morning after side effects. There was no guidance for middle of the night awakening since these drugs are not approved for that indication. Patients who use these drugs should split the tablet to lower the amount of drug taken; it is not clear if they should be split in halves, thirds or sixths. Even then the doses are different from Intermezzo because of the sublingual, rapidly absorbed dose of Intermezzo. The FDA was also concerned during the regulatory process that patients might take Intermezzo once during the night and then reawaken and take another dose. To avoid this with Intermezzo, it is packaged as a unit dose so that the patients if they reawaken will see an opened package and know that they have taken the drug. With Ambien or Ambien CR there is no such packaging and if the patient is splitting pills there may be additional fragments from the split pill still remaining on the bedside table.
There is no question that Intermezzo is a more elegantly designed formulation of zolpidem that is superior to Ambien and Ambien CR for middle of the night awakening in terms of both efficacy and safety. Still, some investors feel that these advantages don't warrant the price differential of about $5.00 for Intermezzo versus pennies for Ambien and Ambien CR. I personally think that the value added is worth $5.00. As someone who suffers from this condition, I think that paying $5.00 to avoid lying awake in bed for hours and then getting up and going to work groggy is good value. What would you pay to be bright and alert at work for a day instead of being groggy? Also remember that Intermezzo is only taken when it is needed making it much more cost effective.
The Intermezzo Launch
Intermezzo was launched about one year ago and results have been disappointing. Originally, Purdue launched the product using a contract sales force. In 4Q, 2012, it decided to relaunch the product. It is now using its own sales force of 525 reps and in addition is using an aggressive direct to the consumer advertising. The reasons for the disappointing launch aren't clear. My view is that it takes a while to get physicians and patients aware that middle of the night awakening is a disease and that there is a treatment for it. If I am correct, better education of physicians and patients may get back on track. I intend to closely track the results of the launch as measured by prescriptions. The following chart shows prescription trends since the launch to give some perspective.
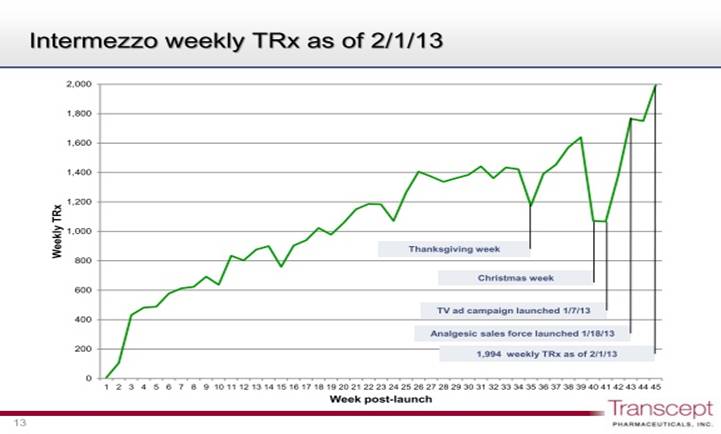
It can be seen that prescriptions slowed during the Thanksgiving to Christmas holiday season, regained the sales trajectory and then dropped as the changeover to the new sales force began in mid-January. The company began its TV ad campaign on January 7, 2013 and the analgesic sales force of Purdue began promoting the product on January 18, 2013. This has caused what looks to be an upward inflection in prescriptions written so that over the last three weeks of this graph, sequential weekly sales have increased at 16% to 18%. For the week ended February 1, 2013, prescriptions written were 1,994. The price per prescription is about $160 net so that the 1,994 prescriptions represent an annualized sales rate of about $17 million.
The issue is just how big of an impact the DTC campaign and Purdue sales force can have. Like everyone else, I will be closely watching the trend of prescriptions. Not as a forecast, but just as a mathematical exercise, if Intermezzo can maintain a 17% sequential weekly prescription increase, it would record prescriptions of 4,193 for the week ending March 29. This would translate into an annualized run rate of $36 million and I think that this would be viewed as encouraging.
Investment Opinion
The tea leaves on degree of the success of the launch are not easy to read. I think that the combination of the superior attributes of Intermezzo and the enhanced marketing program will get the launch back on track. This is key to my continued BUY recommendation on the stock.
Tagged as Transcept Pharmaceuticals + Categorized as Company Reports




