Antares: Thoughts on the Upcoming Otrexup Launch (ATRS, $3.64)
Investment Thesis
Antares is preparing to launch Otrexup in January 2014; it is the first product it has ever introduced and investor attention is keenly focused on the launch. This post is not a comprehensive overview and if you would like to see more in-depth research on other aspects of the Company, I would refer you to my Reports section. In this post, I focus primarily on the preparations that Antares is making for the Otrexup launch.
Probably the most important factor in stock price performance in 2014 will be how the market judges the launch. There is some anxiety among investors because this is the first company launch and also because managed care initiatives have slowed the adoption of new products. Most recent new product launches have been disappointing relative to expectations, even for those that have gone on to gain traction. Some hedge funds have a strategy of shorting into a launch on the assumption that initial launch results will be viewed as disappointing.
Management has avoided giving any numerical guidance on sales potential in 2014 although in the past it initially said that peak sales potential could be in the $100 to $200 million range. With favorable results in a systemic bioavailability trial, management is now guiding to the upper end of that range. I am anticipating a slow initial launch with sales as follows: 1Q, 2014 ($250 thousand), 2Q, 2014 ($1 million), 3Q, 2014 ($4 million) and 4Q, $6 million) resulting in full year sales of $11.3 million. I think that most Street analysts following the stock have comparable first year numbers. My 2015 sales estimate is $45 million.
I have been recommending Antares since my initiation report of December 15, 2011. The basis of my recommendation was a new strategy formulated five years ago to change the company from a business model that was based on licensing its injection technology to corporate partners. The new strategy is to take proven drugs that are off patent and use Antares injection technology to improve the performance of the drug and to provide the intellectual property protection needed to block generic competition. These drugs will be marketed by Antares, not a partner. Its products can be approved by the 505 (b) (2) pathways in which Antares only has to demonstrate bioequivalence to the generic drug.
This strategy makes for a quick turnaround time on product development time. For example, it took three years between the IND filing and NDA approval of Otrexup. For a new chemical entity, this time could very well be 7 to 10 years. The FDA review time for Otrexup was 10 months. Antares believes that its next major product, QS T for testosterone replacement can be launched in 2016. Thereafter, it believes that it can launch one major new product per year for the next five years. If so, this should provide explosive sales and earnings growth over the balance of this decade.
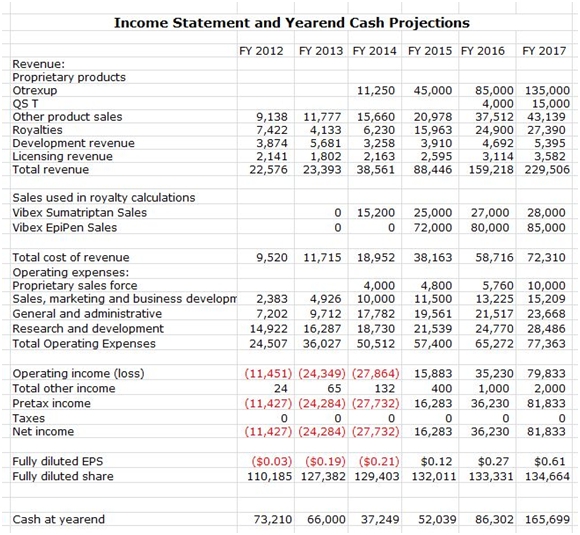
Antares is a complex company with many moving parts, some of which are not addressed in this report. However, estimates for all parts of Antares are shown in the income statement projections shown in the following table.
Otrexup: Product Positioning in the Rheumatoid Arthritis Market
Otrexup is a self-injected dosage form of methotrexate, the gold standard of disease modifying drugs used to treat rheumatoid arthritis. Key opinion leaders that I have heard speak have enormous confidence in methotrexate and virtually all patients who need a disease modifying drug are started on oral methotrexate. Still, there are limitations of oral methotrexate that Otrexup effectively addresses and gives it a significant commercial opportunity.
As rheumatoid arthritis progresses, the physician may need to increase the dosage of methotrexate. Initial does may start at 7.5 mg/mm2/ week. As the dose reaches 15 mg/mm2/ week, the level of drug in the blood plateaus so that increasing the dose does not increase the therapeutic effect. This may force the physician to add on or switch to a biologic drug like Enbrel or Humira to control the disease. There are also some patients who can’t tolerate oral methotrexate because of gastrointestinal side effects.
The product positioning for Otrexup is straightforward. Unlike oral methotrexate, as Otrexup’s dose increases up to 25 mg/mm2/ week, blood levels and therapeutic effect increase directly as opposed to plateauing. This has the advantage of allowing a patient to stay on the trusted methotrexate treatment for a longer period of time postponing the use of biologics.
Antares has indicated that it will price Otrexup at a price of $548 per prescription. Each prescription contains four weekly injectors. This has cost implications as the biologics can cost $15,000 to $25,000 per year versus about $6,600 for Otrexup. If Otrexup can avoid the use of a biologic, a health care plan can save $10,000 to $20,000 per year.
Otrexup enhances and extends the clinical utility of methotrexate through increased bioavailability and improved tolerability. Importantly, Otrexup can be self-injected; it is easy to use and is virtually painless. I have seen some writers dismiss Otrexup as just a new dosage form of methotrexate that has limited medical and commercial potential. This view misses the key clinical that cost and ease of use advantages that meaningfully differentiate Otrexup from oral methotrexate. For an in-depth of the product profile of Otrexup , I would suggest that you refer to the report that I wrote on August 20, 2013.
The Commercial Team Introducing Otrexup
The launch and marketing of a new product entails much more than just hiring salesmen and having them go out and call on doctors’ offices. There are three essential components of a commercial team: the salesmen and their district managers, the national account managers who negotiate access to managed care formularies and the medical science liaisons who educate the medical and managed care communities about the benefits of the drug.
Antares has been planning the Otrexup launch for about 18 months. At this point, the Company has three district sales managers in place. They have received applications from over 500 candidates and are currently interviewing prospects for sales positions. The goal is to hire 25 salesmen and have them in place by Thanksgiving before the actual launch in January. The sales force will focus on the 2,500 physicians that write 80% of prescriptions for oral methotrexate. This physician universe should quickly grasp the clinical value and cost benefit of Otrexup in patients who have not had an adequate response to oral methotrexate.
There are 6 managed care specialists, who have been calling on managed care organizations and third-party payers since June, 2014. They have started to implement a comprehensive strategy to gain access and reimbursement and have had discussions with payers that provide coverage for more than 200 million lives in the United States. Initial access to Otrexup will be at the tier 3 formulary level although several plans have mentioned the possibility of tier 2 placement. Otrexup also met the deadline for Medicare and Medicaid state coverage effective January 1, 2014.
In September, Antares deployed 6 dedicated medical science liaison professionals who have initiated dialogue with many key opinion leaders in rheumatoid arthritis. They are also educating rheumatologists, nurses, patients and caregivers of the therapeutic importance Otrexup.
Antares has built inventory to support a full year of sales. They want to avoid the risk of being out of stock.
QS T: The Next Important Product after Otrexup
On September 16, Antares announced that the first patients have been dosed with its injectable QS T product for males with low testosterone levels or low T. This is a dose-ranging study that is designed to evaluate testosterone administered weekly by subcutaneous injection at doses of 50 milligrams and 100 milligrams. Testosterone is a highly viscous substance that is difficult to deliver by injection. A major advantage of QS T is that it administers the dosage in less than 5 seconds.
Data seen in the first few patients has been encouraging. The therapeutic goal of QS T is to avoid the peaks and valleys of blood levels seen with most other injectable formulations and provide a consistent blood level comparable to gel formulations. This has been seen in patients treated so far.
The complete results of this first study should be available in 1Q, 2014. If the results are encouraging, pivotal clinical studies can be started in 2014, an NDA can be filed in 2015 and QS T can be launched in 2016.
Intellectual Property Protection
Antares’ products are based on generic drugs that do not have the protection afforded by composition of matter patents. However, the Company has a comprehensive strategy for patenting drug formulations and its injectable technology. During the third quarter conference call, the Company mentioned that it had received three new patents on Otrexup in the last few months and that Otrexup is now protected by at least 7 different patents going out as far as 2030.
These patents prevent competitors from copying the injectors and formulations. It does not prevent them from developing their own formulations and injectors. However, any such competitive product would have to be sold as a branded product and could not be interchanged with Otrexup as is the case with generics.
Methotrexate Is a Valued Drug
Methotrexate is considered to be the gold standard therapy for rheumatoid arthritis patients who need a disease modifying agent. It is the most important and valued drug in the rheumatologist’s drug armamentarium. I have listened to numerous presentations at conferences by key opinion leaders in rheumatology and am always struck by the confidence that they have in this drug.
Rheumatoid arthritis patients generally start on drugs such as aspirin, naproxen and ibuprofen that treat the pain and inflammatory symptoms of the disease. As the disease worsens, they progress to disease modifying agents that treat the cause of the disease and the first drug used is almost always methotrexate. If patients fail to respond to methotrexate, physicians turn to newer biologic agents such as Enbrel, Humira and Remicade. Methotrexate may be used alone or in combination with anti-inflammatories and biologics. In total, it is estimated that methotrexate is used in 70% or more of rheumatoid arthritis patients who need disease modifying agents. It is also used to treat psoriasis and other autoimmune diseases.
Methotrexate was first developed as an anti-cancer agent in the late 1940s. In cancer, its mode of action causes the same effect as many chemotherapy drugs; it inhibits the synthesis of DNA in rapidly dividing cells. It has a different mode of action in autoimmune diseases like rheumatoid arthritis in which it blocks the proliferation of T-cells that play an active role in these diseases. This activity was ignored for many years, but beginning in the mid-80s, this effect became more broadly recognized and methotrexate began to be used widely for the treatment of autoimmune diseases.
Methotrexate for rheumatoid arthritis is most commonly given as oral tablets once a week. It is usually started at 7.5 mg/mm2/week and then titrated up to as much as 20 to 25 mg/mm2/week. The absorption of methotrexate can be erratic as the dosing is increased and this can be a problem getting patients titrated to the right dose.
Gastrointestinal side effects such as nausea are troublesome for somewhere between 20% and 50% of patients when initially treated and can prevent dosing to the appropriate level. In some patients these side effects disappear on their own or may be controlled by lowering the dose or spreading the dose out over the course of a day. Some patients take their dose on Saturday so that the side effects do not interfere with the work week.
Otrexup Product Description
Otrexup is a disposable single, self-administered injection intended for once weekly administration of methotrexate. It is injected sub-cutaneously (under the surface of the skin) using Antares’ Vibex Medi-Jet auto injector device in one of four dosages of 10, 15, 20 and 25 mg/mm2/week. (mm2 refers to the estimated skin surface area of the patient being treated). These dose volumes are small and can be comfortably injected in the leg or the abdomen. Otrexup delivers the dose in about half a second, but patients are told to hold the device on the skin for about 3 seconds to make sure that they complete administration of the dose. There is minimal pain on injection. Antares ran a study in which patients were asked to evaluate the pain of injection on a scale of 0 to 100 where 0 represented no pain and 100 represented maximum pain. The Vibex Medi-Jet device used with Otrexup was ranked between 2 and 3.
The clinical program had to show that patients with moderate to severe impairment of their hands-which commonly occurs in rheumatoid arthritis patients-, could safely administer Otrexup. Antares conducted a usability study which showed that 98% of RA patients were able to effectively use it in a home setting. The instruction for use manual provided with each injector was found by 100% of patients to correctly instruct them on how to use the device as intended.
Self-administration of Otrexup is a three step process that takes about three seconds. Patients self-administer the drug by first removing a cap and unlocking the safety by flipping it up. They then touch the collar of the device to the skin to trigger the injection; there is no button to push or any other action required by the patient. The patient never sees the needle which reduces patient apprehension and therefore improves compliance. Otrexup is a well-designed system and contrasts with currently used injectable devices in the US that require removing the drug from a vial with a needle and syringe and then injecting intramuscularly. With intramuscular shots, it is possible to hit a major blood vessel leading to some form of embolism, but there is little risk of this with a subcutaneous injection.
Safety is an important issue as methotrexate was originally developed as a cancer drug with some potentially dangerous side effects and physicians don’t want to inadvertently expose other members of the family to the drug. This injection device is self-contained and delivers 99.99% of the dose of methotrexate leaving little drug residue in the device. In addition, a locking needle shield reduces the risk of any accidental needle sticks following usage.
Tagged as Antares Pharma Inc., ATRS, methotrexate, otrexup, rheumatoid arthritis + Categorized as Smith On Stocks Blog