Northwest Biotherapeutics: My Thoughts on the New Data from the Phase 1/2 Trial of DCVax Direct (NWBO, Buy, $6.91)
Critical Issue in Interpreting the Data
Northwest just recently presented length of survival data for 39 patients evaluated in its phase 1 trial of DCVax Direct. . These patients were suffering from 13 different y types of solid tumors. The common characteristic was they were in the terminal phase of their disease and their tumors were now inoperable; many had been previously operated on only to have the tumor re-emerge. They had exhausted all treatment options and the next likely step was hospice. Such patients have widely metastasized, aggressive tumors that are growing rapidly and because there is no treatment to slow disease progression, their condition declines rapidly. These were the sickest of the sick cancer patients.
Evaluation of efficacy is made difficult because there was no control group in the study. This was in part because the objective of this first in human trial was primarily to evaluate safety as is almost always the case in phase 1 cancer drug studies. Because there was no control group, there is no hard data on what expected length of survival might be in this group if they were not to receive DCVax Direct. In speaking with oncologist contacts, they state that these patients generally die in a matter of months. (As an anecdotal footnote, one of my friends died in three months after he exhausted his final treatment option in metastatic colorectal cancer.)
Based on these anecdotal comments and without hard data, I think that an estimate of six months survival in this patient group may be a reasonable estimate for expected median overall survival for patients not receiving DCVax Direct. I would urge investors to ask their oncologist friends or acquaintances as to whether they think this is reasonable. If investors were to accept six months (or even twelve months) as being a good estimate for expected median overall survival in this group, the results from this phase 1 trial can only be viewed as extraordinarily good. We obviously need more extensive data from upcoming phase 2 trial to make more definitive conclusions and I am sure that critics (short sellers) will come up with numerous criticisms of this hypothesis with the heterogeneity of the group and lack of a control group heading the list.
Introduction
CEO Linda Powers presented new data on the 39 evaluable patients from the phase 1 trial of DCVax Direct in inoperable tumors at the SMi 4th Annual Cancer Vaccines Conference in London on Wednesday, September 16, 2015. The full presentation can be viewed at this link and I would urge every interested investor to take the time to listen to it.
This new data was also presented at another forum on the same day. This was a poster presentation on DCVax Direct at The Inaugural International Cancer Immunotherapy Conference: Translating Science Into Survival, which was held in New York City. The title of the poster was “Cytokine Production by Human Dendritic Cells when Administered Intratumorally Correlates with Clinical Outcome in Subjects with Advanced Cancers”. Click on this link to view the full poster.
This note goes over my key takeaways from this new data set.
Executive Summary of Investment Implications of this New Data
I view the new data on 39 patients evaluable treated in the phase 1/2 trial of DCVax Direct as quite encouraging. This is with the significant caveat that it was an open label, phase 1/2, first in human trial. I think the proper characterization is that there are strong signals of a very meaningful impact on survival in a desperately ill, terminal cancer patient population. However, we will need additional data from upcoming phase 2 trials before we can have high confidence that the drug is truly producing an important survival effect. That said, the survival data was quite encouraging to me.
The primary objective of this trial as is the case with most phase 1 trials is to determine if there are side effect issues and here the results were excellent. The safety profile for the drug appears very good in an absolute sense and benign in comparison to chemotherapy and other currently used (targeted therapy) drugs. Conclusions to be drawn on efficacy have a lower level of confidence, but are certainly encouraging.
The M.D. Anderson endorsed poster provided extensive data on the duration of response for a heterogeneous group of 39 patients treated who were suffering from 13 different cancer types. These were terminal cancer patients with inoperable solid tumors. They had exhausted all treatment options and most were headed to hospice. Based on the opinion of some key opinion leaders with whom I have interacted, I believe that median overall survival for these desperately ill patients could be about 6 months (half survive six months) if cared for with current treatment which is basically supportive care (not drugs). Ask any oncologist you know what they think expected survival would be for patients in the terminal stages of inoperable metastatic melanoma, sarcoma, pancreatic and colorectal cancer might be. I think their answer will confirm what I am saying. Still, I must emphasize that my estimate is not backed by hard data.
If my estimate that six months or so is the expected time for median survival in this heterogeneous patient population is anywhere near correct, DCVax Direct appears to be providing a significant benefit as shown below:
- 9 patients (23%) have survived 18 months or longer,
- 13 patients survived 12 to 18 months or put another way, 22 patients (56%) have survived 12 months or longer,
- 9 patients survived 6 to 12 months or put another way 31 patients (79%) survived longer than six months,
- 8 patients (21%) survived less than six months.
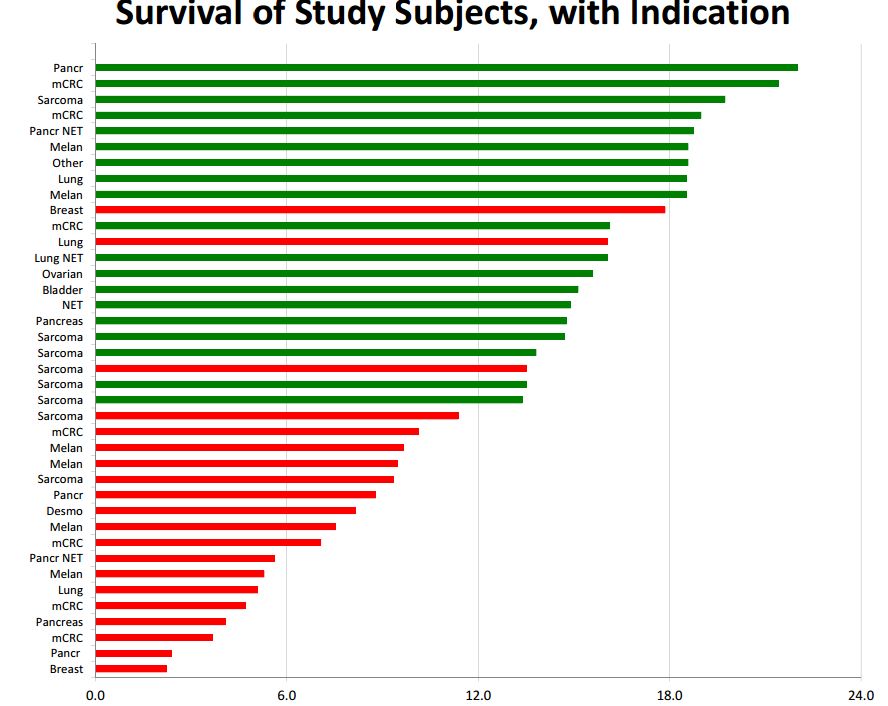
Of the 22 patients who survived for 12 months or more 20 ae still alive, which means that the results already seen can only improve over time. Here is a chart of the data on length of survival. Green indicates that the patient is still alive and red indicates death. The x axis has length of time from the beginning of treatment. The y axis shows the type of cancer treated.
We are in the very early stages of developing new immunotherapy drugs as only the cancer vaccine Provenge and the checkpoint inhibitors Yervoy, Opdivo and Keytruda have been approved. However, one of the hallmarks seems to be that about 20% to 30% of patients experience a remarkably long duration of effect. If we take 12 months or more as a measure of long duration of effect then 56% of DCVax Direct patients experienced a long duration of effect and if we take 18 months or longer, 23% have had a long duration of effect. Hence, it appears that DCVax Direct may have this same hallmark characteristic of a dramatic increase in survival in a subset of patients. It will be extremely interesting to follow the progress of the 20 patients cited above. As I noted, it can only improve over time.
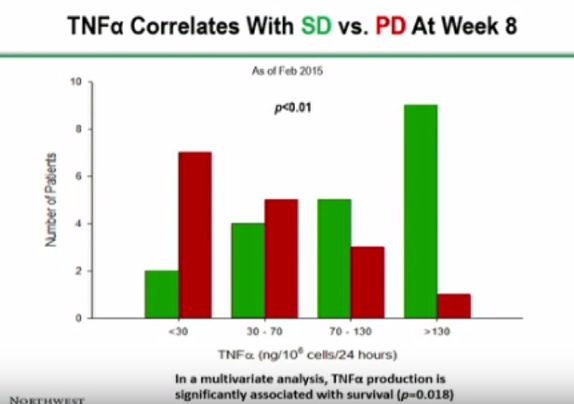
The poster also provided some more esoteric data which requires some basic understanding of immunology to appreciate. This strongly indicates that DCVax Direct is boosting the immune system response to cancer. This is the objective of DCVax Direct treatment. Still other data suggests that there may be markers which could help predict which patients might best respond to therapy.
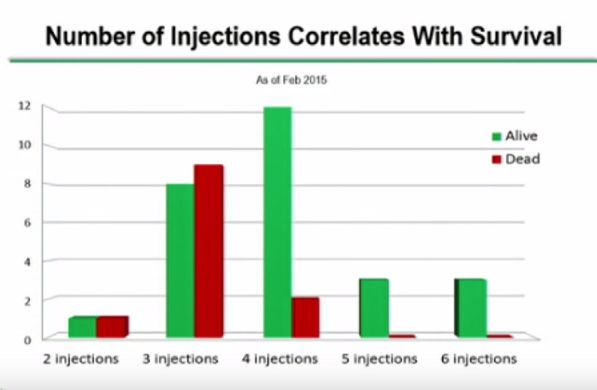
A strong case can be made that the way DCVax Direct was administered in this trial may have produced poorer results than what may be seen in future clinical trials. There was only one tumor site injected in all patients and in future trials the Company has said that up to five different sites may be injected. The complete protocol for this trial called for six injections over 8 months, but I have not seen data as to how many patients received all six injections. Future trials may be longer and with more (many more) injections. It is also the case that the number of cells per injection may have been low in some patients as the first patient treated were given low doses that were then titrated higher in later patients treated. All of this could have the effect of making DCVax Direct results look worse than what we might see in future trials where there will be more than one tumor site injected and all patients will receive what is deemed to be a more optimal number of cells per injections and also number of injections. .
I am very encouraged by these results although I would urge investors in Northwest Biotherapeutics from running any victory laps. I look forward to seeing the updated results from this trial from time to time in the future. The survival results look good, but they have the potential to look even better in the future. The Company will begin phase 2 trials in the immediate future. At this time, I am uncertain as to the design of these trials and the timelines for when we might see topline results. When they do become available, we should have a meaningful insight into the therapeutic potential of DCVax Direct.
From an investment standpoint, there is good reason to believe that DCVax Direct should be considered as an opportunity of the same (or greater) magnitude as DCVax-L. There are now two shots on goal for Northwest. If the DCVax-L trial were to fail, there could still remain a strong investment story. I can see subscribers looking at this and saying oh oh, Larry is getting worried and is hedging his bets on DCVax-L. This is not the case. Clinical trials carry a significant risk of not meeting their endpoints because of the design or execution of the trial and I have consistently made this point. I have seen numerous cases in which drugs have failed to meet endpoints in an early trial and ultimately are shown effective in later trials. This is just the inherent risk in clinical drug development. So in short, I am not signaling any change in my attitude on DCVax-L. Rather, I have become more optimistic on the potential for DCVax Direct.
New Data on DCVax Direct Confirms Earlier Releases of Data
One very important point that I want to make is that the two lead authors on the study which was the subject of the poster presentation were Vivek Subbiah and Ravi Murthy, who were the lead clinicians on the DCVax Direct trial that was done at M.D. Anderson. Both are M.D. Anderson cancer physicians. Other physicians from UCLA were also co-authors of the paper. The reason I emphasize this is that Adam Feuerstein of The Street.com has argued that there is a rift between M.D. Anderson and Northwest which some investors have taken to mean that M.D. Anderson does not support the trial nor data resulting from it. Obviously, this is incorrect.
Feuerstein has gone on to state that earlier data released by the company on DCVax Direct was misleading and promotional. The first release of data on the phase 1 trial was reported in a press release on May 15, 2014. Feuerstein immediately attacked this in a blog which was one of 29 negative blogs on Northwest that he wrote in 2014 and 2015. He stated that the data was based on hospital case reports gathered by Northwest (actually its CRO) rather than data compiled by the investigators. He went on to state that this was somehow unethical although his reasoning for this conclusion is unclear. There was an implication that the data presented was “cherry picked” and incomplete and was an effort on the part of Northwest’s management to promote the stock. This led some investors to be concerned that the data was incomplete, meaningless and promotional. This just published poster gives substantial detail on the phase 1 trial and substantiates and enhances the earlier data released by Northwest. The data just presented in this trial is much more extensive, more mature and of course is vetted by M.D. Anderson physicians. It unquestionably shows that the earlier releases of data by Northwest were accurate portrayals of data from the trial.
Remember that phase 1 trial data is much less definitive than phase 2 and 3 trials. That said, in the context of a phase 1 trial the data presented is rich and detailed and allows intriguing insight into the potential for the drug. It is clear that the phase 1 data does have some encouraging information on safety, patient response to the drug, identification of biomarkers to perhaps identify those patient who will best respond to therapy and insights into immune system effects (cytokine release) that give some insight into mechanism of action. For a phase 1 trial, this is an information rich data release. I think that we can dismiss Feuerstein’s suggestion that the data earlier released by Northwest was contrived and promotional unless he somehow feels that M.D. Anderson is also promoting the stock of Northwest.
In recent weeks, over 10 law firms have filed notice that they are soliciting investors to join as plaintiffs in a class action law suit. Each of these firms have claimed that the earliest data released by Northwest on the trial was somehow conjured up, inaccurate and misleading all with the intent of management to promote the stock. Each law firm cited the work of Feuerstein as the basis for this allegation. The poster just presented on the phase 1 trial clearly shows that what was presented earlier was more than borne out by this more complete data set. It confirms and significantly enhances the information that was released by Northwest in 2014. It is extremely distressing to see law firms apparently acting in concert with short sellers to try to drive the stock price down.
Further Thoughts on the Phase 1 Data
Putting the Phase 1 trial in Perspective
In a phase 1 trial, the primary goal is to show that the drug can be given safely and this has been shown with DCVax Direct. Relative to chemotherapy and most targeted therapies, it has a benign side effect profile and this is not trivial. From this standpoint, the trial was quite successful.
There can be signals of efficacy seen in phase 1 trials and insights into the way to dose the product. The dosage in the case of DCVax Direct is based on number of injections, number of cells injected and interval between injections. Such dosing measures are critical to effective use of the drug. Clearly, larger (maybe randomized, maybe not) trials are needed to determine the efficacy and correct dosing of the product in various types of cancers.
Cancer is extremely heterogeneous. There may be over 100 different cancers and even the same type of cancer can vary in genetic signature from patient to patient. For example, not all breast or prostate cancers are the same and treatment options can be quite different. In solid tumors, the most effective treatment is surgical removal of the tumor so that it can not give rise to metastases. Complete removal of the tumor or tumors is essentially a cure. However, in most cases this is not possible and this is where drug therapy comes in. Drugs can slow the progression of the cancer although only in very rare situations do drugs come close to curing the disease. In almost all cases progression is inexorable.
Perhaps the most important point to make is how severely ill these patients were. The types of patients treated by DCVax Direct were the sickest of the sick. They suffered from inoperable tumors and had failed all approved and usually all experimental drug treatments. DCVax Direct was used to treat 13 different types of cancer occurring in 40 patients in this phase 1 trial. It is difficult to speak categorically as to what the survival prospects of these patients might be, but most of these patients were headed to hospice. Life expectancy was probably a matter of months or weeks rather than years. In this population just slowing or stabilizing the disease is a major importance.
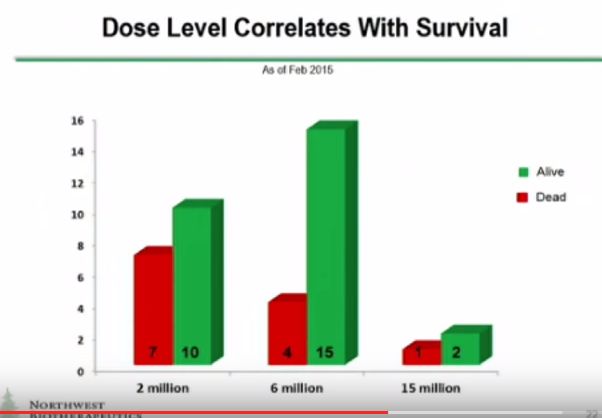
There are also some other things to put in perspective on the trial results which I will discuss shortly. DCVax was only injected into one tumor site. If it eventually comes to market, it is likely that it will be injected into several different tumor masses. The Company has said that up to five tumor sites will be injected in upcoming phase 2 trials. Also three different dose levels as measured by number of cells were given. These were 2 million, 6 million and 15 million cells per injection. Data which I will discuss shortly suggests that the response to the drug improves with the number of cells per injection. It is likely that if the product is approved that the number of cells per injection and the number of injections will be (significantly) greater than that used in phase 1. Perhaps this will lead to better efficacy but that is conjecture on my part. The point I am making is that DCVax Direct might have been used in a less than optimal way. If so, this would make results look poorer than might actually be the case.
Progression Data
Please refer to the chart showing length of survival that I showed earlier in this report. On the y axis, the type of cancer for each patient are shown. By my count, there were 8 sarcoma, 7 pancreatic, 7 metastatic colorectal, 6 melanoma, 4 lung, 2 breast, 1 ovarian, 1 bladder and 3 unspecified types of cancer patients. There is much more to be learned about each of these patients and particularly how those still living progress from this point. We do have detailed data on one pancreatic cancer patient who may have been the first patient treated. See my report National Geographic Special Features DCVax Direct Treatment of Stage 4 Pancreatic Cancer Patient.
The question that everyone is asking is how do these results compare to what might be expected in terminal solid tumor cancer patients. Until, there are larger controlled trials, we can’t authoritatively answer this question. Based on my experience, I think that median survival of 6 months is probably a good estimate, that 12 months is the upper end of expectation for survival and 18 or 24 months is exceptional. If so, I note the following:
- 9 patients (23%) have survived 18 months or longer,
- 13 patients survived 12 to 18 months or put another way, 22 patients (56%) have survived 12 months or longer,
- 9 patients survived 6 to 12 months or put another way 31 patients (80%) survived longer than six months,
- 8 patients survived less than six months
Of the 22 patients who survived for 12 months or more 20 are still alive. It is common in immunotherapy to see about one third of patients experience a long duration of effect. If we take 12 months or more as a measure of long duration of effect then 56% experienced a long duration of effect and if we take 18 months or longer 23% have had a long duration of effect.
I want to caution you that precision on how these results of patients treated with DCVax compare to what might be expected is impossible and perhaps my estimate that expected median survival of six months or so is widely wrong. However, I think that many key opinion leaders would think that I am reasonable. If so, these results are extremely encouraging.
We are in the very early stages of developing new immunotherapy drugs as only the cancer vaccine Provenge and the checkpoint inhibitors Yervoy, Opdivo and Keytruda have been approved. However, one of the hallmarks seems to be that about 20% to 30% of patients experience a remarkably long duration of effect. If we take 12 months or more as a measure of long duration of effect then 56% of DCVax Direct patients experienced a long duration of effect and if we take 18 months or longer, 23% have had a long duration of effect. Hence, it appears that DCVax Direct may have this same hallmark characteristic of a dramatic increase in survival in a subset of patients. It will be extremely interesting to follow the progress of the 20 patients cited above. As I noted, it can only improve over time.
The poster also provided some more esoteric data which requires some basic understanding of immunology to appreciate. This strongly indicates that DCVax Direct is boosting the immune system response to cancer. This is the objective of DCVax Direct treatment. Still other data suggests that there may be markers which could help predict which patients might best respond to therapy.
A strong case can be made that the way DCVax Direct was administered in this trial may have produced poorer results than what may be seen in future clinical trials. There was only one tumor site injected in all patients and in future trials the Company has said that up to five different sites may be injected. The complete protocol for this trial called for six injections over 8 months, but I have not seen data as to how many patients received all six injections. Future trials may be longer and with more (many more) injections. It is also the case that the number of cells per injection may have been low in some patients as the first patient treated were given low doses that were then titrated higher in later patients treated. All of this could have the effect of making DCVax Direct results look worse than what we might see in future trials where there will be more than one tumor site injected and all patients will receive what is deemed to be a more optimal number of cells per injections and also number of injections. .
I am very encouraged by these results although I would urge investors in Northwest Biotherapeutics from running any victory laps. I look forward to seeing the updated results from this trial from time to time in the future. The survival results look good, but they have the potential to look even better in the future. The Company will begin phase 2 trials in the immediate future. At this time, I am uncertain as to the design of these trials and the timelines for when we might see topline results. When they do become available, we should have a meaningful insight into the therapeutic potential of DCVax Direct.
From an investment standpoint, there is good reason to believe that DCVax Direct should be considered as an opportunity of the same (or greater) magnitude as DCVax-L. There are now two shots on goal for Northwest. If the DCVax-L trial were to fail, there could still remain a strong investment story. I can see subscribers looking at this and saying oh oh, Larry is getting worried and is hedging his bets on DCVax-L. This is not the case. Clinical trials carry a significant risk of not meeting their endpoints because of the design or execution of the trial and I have consistently made this point. I have seen numerous cases in which drugs have failed to meet endpoints in an early trial and ultimately are shown effective in later trials. This is just the inherent risk in clinical drug development. So in short, I am not signaling any change in my attitude on DCVax-L. Rather, I have become more optimistic on the potential for DCVax Direct.
Other Charts Presented in the Poster
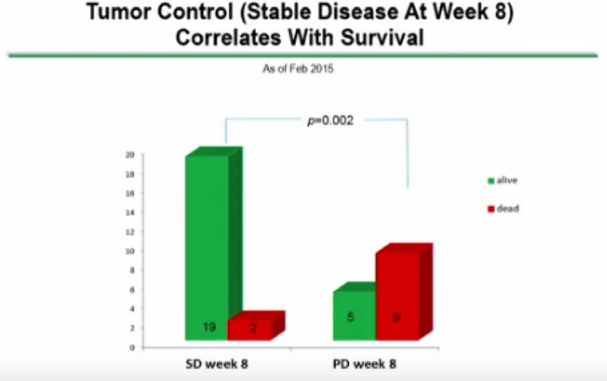
The length of survival data is the most interesting chart. However, the balance of the poster presents charts that show interesting data (some a bit esoteric) and I am not sure that I understand it all. For those with good understanding of immunotherapy, I recommend that you look at the poster presentation. I am not going to try to explain every chart. Here are the charts that I found most interesting.
Tagged as Data on 39 patients from phase 1 trial of DCVax Direct, dcvax direct, Northwest Biotherapeutics Inc., NWBO + Categorized as Company Reports





Larry, As you said these results are very encouraging. Do you have any news on the current financial status of NWBO? Last I heard they were running quite low on cash. It would sure be nice to get that behind them.
The more success that DCVax Direct shows in these Phase I/II trials the more difficult it will be to enroll patients in a randomized Phase III trial. Who is going to volunteer for a trial where there is a 50% chance of being assigned to a placebo arm when the available treatment has been shown to be highly effective?
I would think that patients in the DCVax – L trials are getting a little nervous about their assignment status when they read about the results being obtained in the DCVax Direct trials.
Thanks, Larry, for your really extensive analysis of the DCVax Direct trial data. I guess I was premature in thinking that there was not much new in the data released on 16 September.
Regards, Bob Roig
I appreciate the article, especially given the general meltdown in biotech stocks underway.
However, while the news appears to be quite good, there are one aspect that I find frustrating. Specifically, the patient survival chart reflects information available as of September 1, 2015. However, all the other charts are dated from February 2015. It would seem that it would be a relatively simple matter to update those additional tables given the information that the company seems to have available.
Furthermore, in my mind it would seem that only the dosage chart appears to be obviously compelling. All the charts after that seem to be time dependent to a degree or appear not to distinguish correlation from causation.
Focusing on the Number of Injections, It would seem that only 3 of what I guess were 11 eligible patients in February still alive at the 8 month point received the full six injections. So, it does seem that only a small sample of the patients were targeted for the full six injections. So survival bias may not be as important as I first assumed.
I’m obviously a lay person so I’d appreciate if Larry or others could address my concerns.
Al Marshall
The length of survival data is most of the story. It seems extraordinary.
Thank you Larry for your considered analysis of the trial results and what that might mean moving forward…..Money is a huge issue……interim analysis of ‘L’ is a huge issue….You did not touch on the announcement that over 300 patients have been enrolled and screening of :L: is now on hold…(yes, you did publish an article about that), but I would like you to revisit it at some point to give an idea of when the interim analysis of “L” might be forthcoming….Again, thank you for your report….I am glad I am a subscriber and will continue to hold NWBO and “stay tuned” cheers
while screening is on hold, patients continue to be enrolled. I can not come up with a scenario to explain this.
Two points for Bob & Al to consider (hope this helps);
– Bob, you are correct, what terminally ill patient wants to roll the dice and get a placebo and, in turn a death sentence by enrolling in a Phase II or Phase III DCVax Direct trial. That is why, I would think NWBO will probably design the DCVax Direct Phase II trial similar to DCVax-L for GBM patients. The trial uses Progression Free Survival (PFS) as the primary marker rather than Overall Survival. For patients that have GBM or termial phase cancer patients (as defined by Larry above), it makes sense to design trials using PFS as the primary marker instead of OS, as this allows for the ability to treat every patient with the “trial” drug/vaccine. For DCVax-L, a placebo is given to the control group, and DCVax-L to the other group. If any patient in the control group sees their tumor progress, then they are immediately considered an “event” and they are then given the actual vaccine. It allows those in the control group to still get the vaccine and a chance of survival (if the vaccine is working). I would think that this design would be used for DCVax Direct trials as well.
– Al, you are correct, the injection characterization graphs have a February 2015 date. I would assume that this is to evaluate the effectiveness of differences in how the injections are administered. If a Sept 2015 date were used, the graphs may not tell the same story, as there may be several more deaths. However, the graphs are trying to measure/show injection effectiveness, and so it must be taken at some time period. The only way I would improve upon this graph is maybe using patients that live longer than 8 – 12 months in green, and < 6-8 months in red. The graph is trying to show that there may be a correlation of a better response with how the vaccine is administered (or how much). The Sept 2015 may show more dead patients because it is ~ 6 months later.
I do agree with Larry though, it is evident with NWBO, IMUC, and many of the vaccine trials that subsets of patients really respond to immunotherapy. And it will be interesting to see how the FDA administers trial results. Particularly if ~ 26% of patients really respond with longer lives, an progression free survival. These patients could just have more responsive immune systems that respond to the vaccines, rather than other patients. Its like the common cold or flu, everyone's bodies respond differently to colds and flus, and this would be the same with immunotherapy, as it relies on your own body to respond to the vaccine injected into your body. The FDA understands this, and so it will be interesting to see how they rule on some of these phase 3 trials that may come out and show a significant increase in a subset of patients, even though the Overall Survival may not be that different.
What I dislike about the OS as an endpoint, is that the median value is taken instead of the mean value. The difference is that 50% of the patients could have little to no response to the vaccine, and the rest of the 50% could have amazing response, and the median value would show no improvement, but the mean value could show amazing improvement. This is also most likely why NWBO decided to boost their phase III trial size, as this provides the trial with a much larger subset group, which in turn, (if there truly is a significant positive effect of the vaccine on a ~25% subset), strengthens the trial data to show statistical significance more easily and quickly. And when I say easily, I mean if the subset shows significant overall survival, or in this case, progression free survival.
Hope that helps