Cytokinetics: An In-Depth Look at the Potential for Success of Tirasemtiv in the BENEFIT-ALS Trial in ALS Patients (CYTK, $9.50) (Subscribers Only)
Purpose of This Report
The biggest clinical trial event in all of biotechnology for 2014 could be the reporting of phase 2b results from the BENEFIT-ALS trial of tirasemtiv in ALS. This in-depth report analyzes earlier phase 1 and 2 results for tirasemtiv and how they might be predictive of a positive outcome in BENEFIT-ALS. In addition and extremely importantly, blinded data from 670 BENEFIT-ALS patients treated through November 2013 was recently presented. This suggests that tirasemtiv is having a positive therapeutic effect in BENEFIT-ALS, but until the data is unblinded we can’t know if this is actually the case and whether this is clinically significant.
I have gone through this data described in this report in some detail and my broad conclusion is that tirasemtiv does have a biological effect that can slow the inexorable decline in physical status of patients. Importantly, it seems to improve respiratory function which is critical as most ALS patients die of respiratory failure or from complications such as infections that stem from use of mechanical ventilators. This is suggestive that BENEFIT-ALS will be successful, but until the data is unblinded we can’t really determine if this is the case.
Please read the investment thesis of this report very carefully. I believe that the stock of Cytokinetics (CYTK) offers an extraordinarily attractive reward to risk ratio and this is the basis of my recommendation. However, drug development and outcomes of clinical trials are inherently risky investments and you could suffer a quick and significant loss of capital if the BENEFIT ALS trial is a disappointment or fails. An investment in CYTK should be a part of a diversified portfolio that takes such risk potential into account.
Incidence of ALS
ALS is an orphan disease with a large unmet medical need. There are 25,000 people living with ALS in the US; the incidence in men is 1 in 500 and for women it is 1 in 800. Prevalence is low because patients die so quickly. The annual incidence of ALS is about the same as another neuromuscular disease, multiple sclerosis, but because of lesser mortality there are 450,000 patients living with multiple sclerosis. If therapy could allow ALS patients to live longer the prevalence would be higher.
Patients’ life spans can be extended modestly by supportive care, but there are no effective drugs. The ALS community is very aware of tirasemtiv. It is important for ALS patients to know that there is something being done, that there is some hope given the numerous prior failures and the absence of any effective drugs. BENEFIT-ALS results are keenly anticipated.
Rilutek is the Only Approved Drug for ALS
There is only one drug approved for the treatment of ALS; Sanofi’s (SNY) Rilutek (riluzole), which was approved in the mid-1990s. In younger populations, it has a modest effect on survival of about two to three months. However clinicians report that it has no effect on quality of life or activities of daily living. Even with these poor or limited product characteristics it is used in 2/3 of ALS patients and is reimbursed by Medicare. There are about 75 centers of excellence in the US that treat about 50% to 60% of the US patient population.
Rilutek was studied in two clinical trials. The endpoint of these trials was the need for tracheostomy or death. Interestingly, it did not achieve statistical significance in the first study on its endpoint based on the log rank test (p=0.12) prospectively defined. In the second study the log rank test p value was 0.076. When both trials were combined, it did achieve statistical significance by the Wilcoxon statistical test (p=0.05) and the FDA approved it on this basis. Rilutek appears to increase median survival by 90 days in younger populations.
The most recent information on Rilutek came from the failed Biogen Idec study of dexapramizole in which Rilutek was used as a control arm. It supported the previous data that shows that Rilutek extends survival by two to three months. The failure of dexapramizole was the latest in a long line of failed drug development attempts in ALS. Despite numerous attempts, over the last 20 years since Rilutek was approved no other drugs for ALS have succeeded in ALS.
Some Background on Tirasemtiv
Because we don’t yet have sufficient data to accurately assess the benefits and risk of tirasemtiv in ALS, we can only make guesses at its potential. The first thing to note is that the mode of action of tirasemtiv is to improve the response of muscles to neuronal input. It does not address the underlying cause of ALS which is progressive degeneration of nerves in the spinal column.
This does not mean that tirasemtiv will have no effect on duration of life. By improving strength of muscles involved in breathing, it has the potential to improve length of survival. Unlike Rilutek, it could improve quality of life by improving muscle responses that control speech, salivation, swallowing (eating) and a host of fine motor and gross motor functions. This would be of great benefit in improving the quality of life for ALS patients.
This note goes through the phase 1 and 2 data on tirasemtiv. These were not long term studies and we have to be careful about drawing conclusions. BENEFIT-ALS is the first truly large and longer duration randomized trial that can begin to define the therapeutic profile of tirasemtiv. The data suggests to me that over short periods of 14 to 21 days that tirasemtiv in the earlier phase 1 and 2 trials stabilized ALS or even improved the condition of patients. In addition, blinded data from 670 patients treated in BENEFIT-ALS through November 2013 suggests that tirasemtiv is having a positive effect.
The dose limiting side effect of tirasemtiv is dizziness or light headedness that appears transient and shouldn’t meaningfully affect the development and adoption of the drug.
A key question is how durable the effect of tirasemtiv may be. The length of the BENEFIT-ALS trial is three months, which will give a better insight into durability of effect. The next question is how meaningful tirasemtiv will be to quality of life and duration of life. In oncology, extension of median overall survival of four months is considered a significant therapeutic advance. Perhaps, we can use that as a standard for tirasemtiv.
In designing BENEFIT-ALS, Cytokinetics consulted with key opinion leaders who concluded that slowing the rate of decrease in the ALSFRS-r scale (this scale is a measure of the status of ALS patients and is explained later in this report) by 20% to 25% over a period of months would be clinically meaningful and this is the endpoint of the BENEFIT-ALS trial.
BENEFIT-ALS Trial Status
The BENEFIT-ALS trial completed enrollment of 711 patients in 4Q, 2013. As of a conference call in early February, 400 of those patients had completed the 12 weeks of double-blind treatment. There are still patients on treatment and the Company hopes that the last patient treatment will complete by the end of February and last patient follow-up will complete by the end of March.
The goal is to present the data in April at the Annual Meeting of the American Academy of Neurology. An abstract has already been submitted by the lead investigator on the trial, Dr. Jeremy Schefner on the expectation that the full data set will have been analyzed by then. The AAN has accepted the abstract and if it is ready, it will be the subject of a podium presentation.
BENEFIT-ALS has 80% power to detect a lesser difference in treatment effect versus placebo from baseline of 1.18 on the ALSFRS-r scale at 12 weeks with a p less than 0.05. The assumption based on historical data is that placebo patients will experience a decrease of 0.9 points per month. If this assumption is correct, the placebo patients will experience a 2.7 point decline in ALSFRS-r over the course of the trial and for tirasemtiv to be successful, it will have to hold patients to a 1.6 point decline or less.
This is about a 35% to 40% improvement in the rate of decline. Cytokinetics surveyed KOLs who responded that they thought that a 20% to 25% improvement would be clinically meaningful. If tirasemtiv achieves this 1.18 point differential, it will be the first time that any drug has shown a statistically relevant improvement on the ALSFRS-r scale.
Potential Commercial Value of Tirasemtiv
We will have to await the completion of the BENEFIT-ALS trial and its interpretation by key opinion leaders and ultimately the FDA before we can truly assess the medical value of the product. However, let’s assume that the conclusion ultimately reached is that tirasemtiv is an effective and valuable drug. The community of physicians who treat ALS is small and they are all very much aware of tirasemtiv as are the ALS patients. I would expect a very quick uptake of the drug.
With its modest efficacy, Rilutek is used by about 2/3 of the 25,000 ALS patients or roughly 17,000 patients. Based on prices charged for innovative drugs recently introduced for various types of cancers, cystic fibrosis and multiple sclerosis, tirasemtiv could be priced in an annual range of $50,000 to $100,000 depending on its profile. Gilead (GILD) recently released its new product for hepatitis C, Sovaldi, at a price of $84,000 for a 12 week course of treatment. Using a $50,000 price estimate and an estimated addressable patient population of 17,000, this translates into potential peak sales in the US of $850 million; international potential could be the same or larger resulting in potential worldwide peak sales of $1.7 billion.
So what is the value of this to Cytokinetics shareholders? The stock market usually values sales of important new drugs at anywhere from four to seven times sales. Using $1.7 billion of sales and a multiplier of four would suggest a market value of $5.2 billion for tirasemtiv based on peak sales. Based on 43.6 million fully diluted shares, this translates into $119 per Cytokinetics share.
There is a host of other neuromuscular diseases in which tirasemtiv could potentially be of value such as myasthenia gravis and Duchenne muscular dystrophy. These increase the sales potential of tirasemtiv. You can see why I started this report by saying that results from BENEFIT-ALS could be the event of the year in biotechnology.
Thinking About the Trial Outcome
I think that there is a reasonable chance that the results of the trial will be an outright success. I also think that if the primary endpoint is not reached with p=0.05 or less, that the data could be sufficiently encouraging that the Company still would move on to a confirmatory phase 3 trial. The base case of the Company is that they will have to do another, confirmatory, phase 3 trial, but in the event that the results in BENEFIT-ALS are spectacular that they could potentially file for approval.
If BENEFIT-ALS is a success, I think we are looking at a $20 stock. If it is a complete failure and the trial results are so bad that the product is dropped, I think that the stock drops in the short term to $3.00 to $4.00. There are many in-between scenarios depending on the data in which the Company proceeds to a phase 3. In the latter case, I think there would be significant initial pressure on the stock as the market absorbs the news that the primary endpoint was not reached with statistical significance, but the stock could then bounce back after more careful analysis.
Cytokinetics has another major asset with omecamtiv mecarbil that is being studied in heart failure. If omecamtiv is successful in phase 3, it meets one of the largest unmet medical needs in medicine and I see it as a multi-billion dollar drug. One interesting aspect of this investment situation is that Amgen, the licensee of omecamtiv mecarbil, is committed to paying Cytokinetics $300 million or so of milestones before commercialization. Much of this will probably relate to the filing of the NDA and approval of the NDA, but there could be a substantial in-flow before then. I would speculate that there could be milestone payments based on the decision to move into phase 3 and perhaps when the first and last patient is dosed. I can only guess at the amounts, but they could be in the $50 to $75 million range in 2015 and give CYTK a solid financial footing into 2016 and perhaps 2017.
If tirasemtiv fails and the stock goes to $4.00 or some such price, I would view it as a buying opportunity based on the potential for omecamtiv mecarbil. If Amgen takes omecamtiv mecarbil into phase 3 in 2015, I could see the stock at $15.00 on this basis alone and with no value attributed to tirasemtiv. If omecamtiv mecarbil is then successful in phase 3, it could be a multi-billion product and Amgen probably buys the Company for $25 to $30 per share. This is a complex investment scenario but it is an excellent asymmetric upside investment. I suspect that I will be doing a lot of writing on CYTK in 2014.
What Happens after BENEFIT-ALS Data is Reported?
Cytokinetics’ base case is that they will proceed to a phase III trial following the completion of BENEFIT-ALS. They are moving forward on this basis and the goal would be to start the phase 3 trial before the end of 2014. In parallel, the Company is also preparing for a commercial launch of tirasemtiv in the US if the BENEFIT-ALS results are spectacular.
In ALS, the unmet medical need is so high that there might be a quicker route to regulatory approval based just on a single Phase 2b study.
There have been no new drugs introduced in the last 20 years and there is enormous pressure on the FDA from the ALS patient community to get new therapy to patients. It is possible that tirasemtiv could be designated as breakthrough therapy that provides for a condensed development program. Cytokinetics has to prepare for the possibility that BENEFIT-ALS will support an accelerated registration while at the same time also planning to conduct a confirmatory phase III trial.
So what would constitute spectacular results in BENEFIT-ALS? To file after a single study they would have to achieve a p value that is meaningfully better than p=0.05, perhaps 0.03 or better. There could not be any internal inconsistencies that might raise any questions such as seeing most of effect coming from one center or a small group of centers. There would also have to be confirming data from secondary endpoints such as SVC, MVV and SNIP which are objective measure of respiratory function.
Assuming the results do not clearly indicate that the drug has failed, they would move to Phase 3. For example, even if they did not hit p=0.05, but only missed by a narrow margin and if the secondary endpoints such as SVC, MVV and SNIP were all confirmatory, they would move forward into Phase 3. The data from BENEFIT-ALS might enable them to better design the phase III for success. For example, they might increase the sample size from 711 patients or narrow the inclusion and exclusion criteria. It is a little early to guess what this Phase 3 trial might look like, but as a guess it might be a trial of six months duration (instead of three months with BENEFIT-ALS) and with 90% power (instead of 80%) to see a treatment effect. This would probably lead to trial of 1,200 to 1,300 patients.
Summary of Blinded BENEFIT-ALS Data and Earlier Phase 2 Results
Dr. Jeremy Schefner, the lead investigator on BENEFIT-ALS provided updates in December 2013 at the international symposium on ALSMMD. These are included in a slide presentation on the Cytokinetics website. This included blinded results from BENEFIT-ALS on 670 patients treated through November 2013 and some double blind data from earlier phase 2 trials.
I have gone through this data in some detail and my broad conclusion is that tirasemtiv does have a biological effect that can slow the inexorable decline in physical status of patients. Importantly, it seems to improve respiratory function which is critical as most ALS patients die of respiratory failure or from complications such as infections that stem from use of mechanical ventilators.
The major caveats I have is that the earlier trials were relatively small and we haven’t seen unblinded data from BENEFIT-ALS. I depend on phase 2 trials that enrolled 49 patients and 27 patients respectively. With such small numbers, there is always the chance that the results are spurious. Also the trials only lasted two weeks and three weeks versus 12 weeks for BENEFIT-ALS. It may be the case that the results will not be durable over 12 weeks.
That said, I think that there is a reasonable chance for success and this is the basis of my recommendation of Cytokinetics.
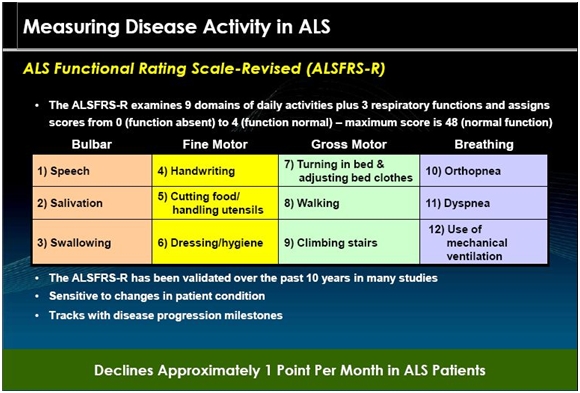
ALSFRS-r
The ALS Functional Rating Scale is the most important measure of the degree of impaired functionality caused by ALS and can also track the progression of the disease. It combines nine criteria involved with daily activities and three involved with respiratory function. It is based upon a subjective measurement by the physician or patient for each function. A score of 0 indicates that there is an absence of the function and a score of 4 means that there is normal functionality. The 12 elements of the scale are shown below:
A normal person would score 48 and a person with ALS would score about 36 a year or so after diagnosis. There is a persistent decline in the ALSFRS-r score of about 1 point per month. Key opinion leaders state that while there can sometimes be a period of stability of a month or two, there is an inexorable decline and sustained improvements just don’t occur. Death usually occurs when ALSFRS-r reaches 16 to 18. Death is almost always the result of respiratory failure or complications such as infections that can occur when breathing must be assisted by mechanical ventilation.
The change in the decline of the ALSFRS-r scale is the primary endpoint of the BENEFIT-ALS trial. This is a validated scale that has been accepted by the FDA in other ALS drug trials to be the acceptable endpoint for registration.
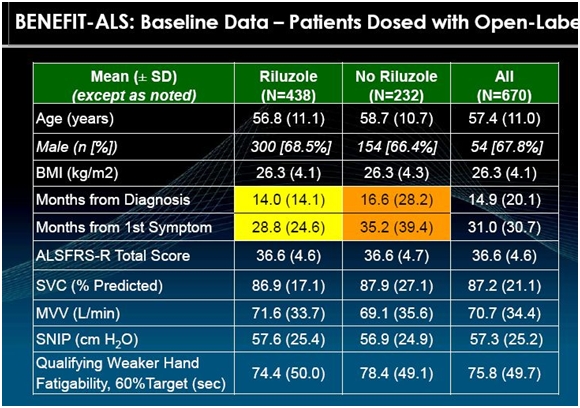
Characteristics of Patients Entering BENEFIT-ALS
The primary purpose of BENEFIT-ALS is to measure the deterioration of patients on tirasemtiv and placebo. It is very important to enroll patients with similar ALS characteristics at the start of the trial (baseline). The following shows the baseline characteristics of 670 ALS patients that had been enrolled through November 2013 in BENEFIT-ALS.
The ALSFRS-r scale is based on subjective measures as I previously explained. Cytokinetics has also used objective measures in its earlier phase II trial as shown in the above table. SVC, MVV and SNIP are measures of breathing and respiratory function. The most important goal of any drug intended for use in ALS is to maintain breathing and respiratory function; deterioration is what kills ALS patients. The following paragraphs briefly explain how these measurements are made.
SVC is short for slow vital capacity. It is the amount of air that can be exhaled after a patient inhales as deeply as possible. A tube is inserted into the patient’s mouth and they take a deep breath and exhale. A spirometer determines the amount of air exhaled (in liters) and compares it to the amount that would be expected from a normal person with comparable physical characteristics. The results are calculated automatically by the spirometer. The calculation is a little more complex than the somewhat simplistic explanation I have given as it involves inspiratory reserve volume, tidal volume, and expiratory reserve volume, but I don’t think it is necessary to get into that type of detail.
MVV is maximum voluntary ventilation. It provides an estimate of the ventilatory reserves that a patient has for physical exercise such as walking or climbing stairs. A spirometer is also used for this test as a tube is inserted into a patient’s mouth and they then take regular breaths in and out over a period of time. An ALS patient has impaired respiratory muscles that are less able to sustain exercise. This measurement is also in liters and the data output is calculated by the spirometer.
SNIP is sniff nasal inspiration pressure. This is a simple way to measure breathing function other than with a spirometer. One nostril is blocked and a clip with a valve is inserted in the other nostril. The patient then takes a maximum sniff and the nasal valve of the patent (open) nostril collapses and the pressure measured beyond the collapsed segment closely reflects esophageal pressure and, therefore, inspiratory muscle strength. This is also performed and calculated with a machine.
Qualifying hand fatigability is a measure of limb muscle strength unlike SVC, MVV and SNIP which measure respiratory function. Skeletal muscles in all people become progressively weaker during periods of activity. Muscle fibers of patients with ALS are easily fatigued, and thus do not respond as well as muscles in healthy individuals to repeated stimulation. This is test of endurance again using a machine to measure the output. The maximum grip strength is determined in the weaker of the two hands. The patient is then asked to hold the grip at 30% of maximum grip strength (as determined by the machine) and the length of time that this can be maintained is then measured and recorded.
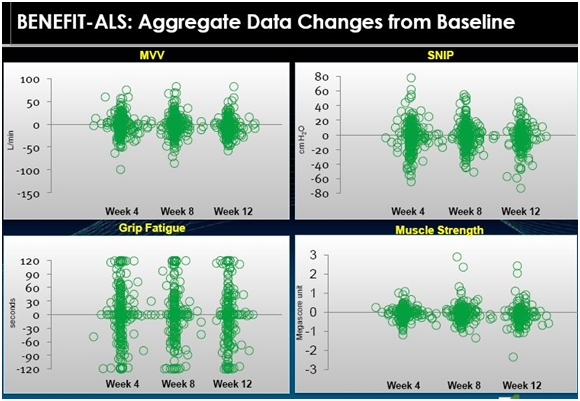
Measurement Outcomes from Blinded Data of 670 Patients in BENEFIT-ALS
In its slide presentations, Cytokinetics put together a slide showing blinded preliminary data involving 670 patients who were treated with either tirasemtiv or placebo in BENEFIT-ALS. The following is a visual representation of the data in which each circle represents an individual patient. The blob of green in the middle of the following displays is because of the overlay of numerous circles (patients).
We don’t know which patients received the drug and which the placebo nor the dose or length of time that patients were on tirasemtiv. This is a mishmash of data and it is hard to interpret as it cannot be assessed with statistical techniques due to the wide variation in the data. Take a look at the following table.
We can only make a visual interpretation of the data and any conclusions are highly circumspect and could be wrong. Let me take a crack at an interpretation.
If we look at MVV as the first example, it appears to me that the chart suggests that the population as a whole does not experience much change. It looks like half of the patients got better and half got worse. Historical data suggests that MVV deteriorates fairly steadily at a rate of 3% to 4% per month. If this is correct, we would expect all placebo patients to show deterioration in MVV. This would suggest that the patients in the above table who showed improvement were on tirasemtiv. Otherwise all of the patient dots would be below the zero line that indicates no change in MVV. The same conclusions could be drawn for SNIP, grip fatigue and muscle strength.
Key opinion leaders suggest that ALS patients may stabilize for a month or two, but the trend is steadily downward. If this is correct, the above tables strongly suggest that tirasemtiv has an effect on the disease. However, I don’t think that we can totally rule out a placebo effect in this data. It is possible that the hope that comes from being offered a new experimental drug could produce a placebo effect.
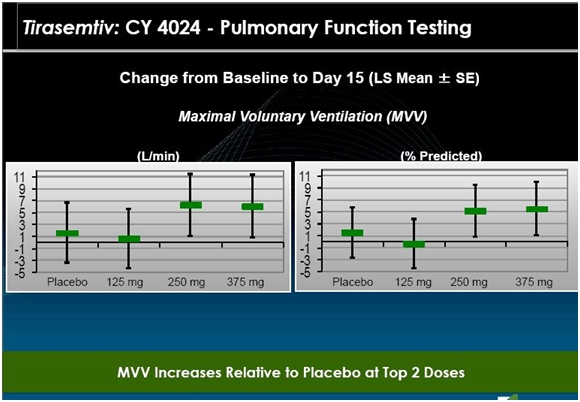
Results from CY 4024, an Earlier Phase 2 Study
This study was broken into two parts. In part A, 24 patients were randomized. All were removed from Rilutek for a period of seven days (a washout period). They were then randomized into four different treatment groups in which they were given placebo or 125 mg, 250 mg or 375 mg of tirasemtiv daily for 14 days. They returned for assessment at days 2, 8 and 15 and were followed up within seven days of their last dose.
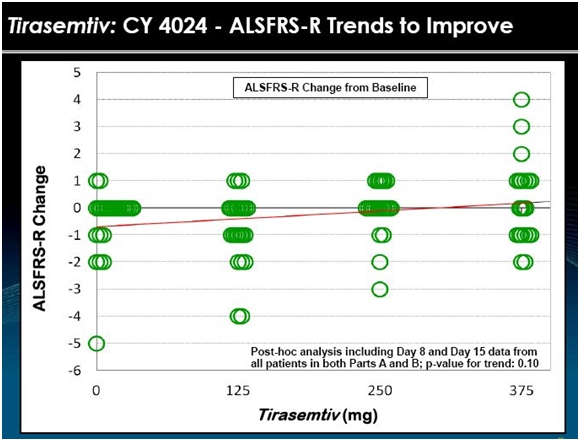
Part B enrolled 25 patients was the same as Part A with the exception that the Rilutek dose was lowered to 50 mg once a day, but continued. Take a look at the following chart.
The data from 24 patients in Part A and 25 patients in part B is shown in the above chart. Each circle is the assessment of the change in ALSFRS-r at days 8 and 15. Visually, the data suggests that patients seemed to do better on tirasemtiv at a daily dose of 125 mg and 250 mg and much better at 375 mg. In BENEFIT-ALS, the daily dosage is 500 mg.
This was a very short term study with a small number of patients so that any interpretation must be made with caution. However, there appear to be just two patients on placebo who had a one point improvement in ALSFRS-r in this two week time frame while about seven patients on the 375 mg dose had an improvement with some showing an impressive 2 to 4 point improvement in ALSFRS-r. The 125 mg and 250 mg doses look similar to or slightly better than placebo. Generally, there seemed to be a dose effect as shown by the trend line. This is always encouraging. If the drug is effective, we would expect more of the drug to produce a more positive effect.
All of this suggests to me that there is a positive biological effect of tirasemtiv in this study in the short time frame of two weeks. It does not give any information on whether the effect would continue over months or years.
The next slide suggests that the effect on MVV was also positive and confirms the improvement in ALSFRS-r. The 125 mg dose was similar to placebo, but MVV improved on both the 250 mg and 375 mg daily dose. This is quite important as ALS patients die from respiratory failure so that the improvement in breathing suggests that tirasemtiv could extend life. Again the major caveat is that this is short term data and a limited number of patients.
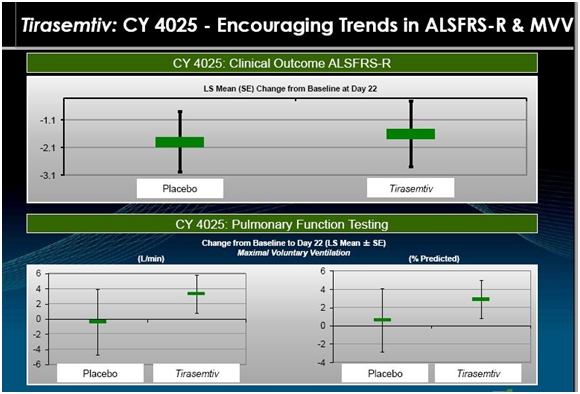
CY 4025; Another Earlier Phase 2 Study
This study enrolled 27 patients who were randomized so that 21 received tirasemtiv and 6 received placebo. Prior to entering the study, their Rilutek dose was reduced to 50 mg a day for seven days. Patients on tirasemtiv were then given 125 mg twice a day for seven days. They were then titrated upward to a daily dose of 375 mg of tirasemtiv (125 mg in the morning and 250 mg in the evening) for seven days. This was followed by a further titration to 500 mg daily (250 mg dose given twice a day) for seven days. Altogether, the 21 tirasemtiv patients were on the drug for 21 days and were evaluated on day 22. The following slide is a representation of outcomes in that study.
The above slide suggests that the rate of decrease in ALSFRS-r was slowed by tirasemtiv. Over the three week period the seven placebo patients had about a 2.0 point decline in ALSFRS-r within a range of 1.0 to 3.0. The tirasemtiv patients had a lesser decrease of about 1.5 within a range of stabile to 2.9. Breathing function as measured by MVV decreased slightly for placebo and increased for tirasemtiv patients.
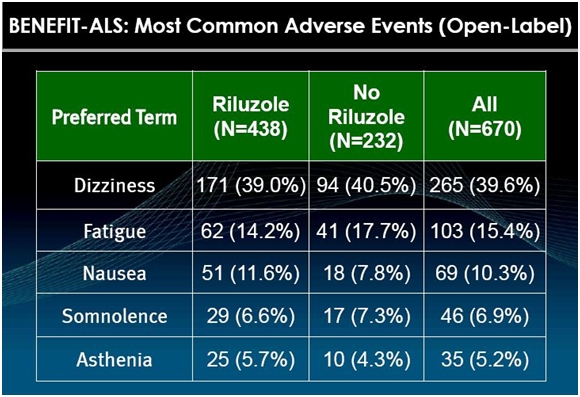
What about Side Effects?
The most prominent side effect of tirasemtiv is dizziness as is shown in the following table.
Physicians who have used the drug say that this side effect makes patients uncomfortable, but the effects are mild and go away after a week or so. Still, this could cause trouble in the BENEFIT-ALS trial if a meaningful number of patients given tirasemtiv were to drop out so that tirasemtiv on an intent to treat basis would be disadvantaged versus control. BENEFIT-ALS is designed to prevent this by giving all patients in the trial a low 125 mg bid dose of tirasemtiv for one week before randomizing them. In addition, patients are gradually titrated upwards to the maximum tolerated dose of tirasemtiv.
After the one week lead in period, the patients who can tolerate tirasemtiv are randomized into a drug group and control group; the duration of the trial is 12 weeks.
Tagged as BENEFIT-ALS, cytk, Cytokinetics, tirasemtiv + Categorized as Company Reports