Neuralstem: Phase 1b Results for NSI-189 are Very Encouraging but It Is Early Days (CUR, Buy, $4.38, For paid subscribers)
Investment Thesis on Neuralstem
I continue with my long standing buy on Neuralstem that is almost entirely based on the potential for its neural stem cell product NSI-566 in the treatment of ALS. Recently, encouraging phase 1b results have been reported for the small molecule drug NSI-189 in the treatment of major depressive disorder. These results are very early stage and came from a small trial, but they are quite encouraging. This adds a new leg to the investment story for Neuralstem that is not reflected in the current stock price.
Background on NSI-189
I think that some background might be helpful in putting in perspective the results of the phase 1b trial of NSI-189 in major depressive disorder (depression). The hypothesis underlying the development of NSI-189 is that aging and disease cause the hippocampal region of the brain to atrophy. The hippocampus is the part of the brain associated with memory, thought processes and psychiatric and cognitive disorders such as depression, Alzheimers and various dementias. There appears to be a high correlation with atrophy of the hippocampus and these diseases.
For many years, scientists had thought there was little or no neurogenesis (growth of new neurons) after birth, but this view is changing rapidly. Research now suggests that limited neuron formation does occur throughout life in the hippocampus. The growing belief is that we humans all have an endogenous supply of neural stem cells that provides for some replenishment of the hippocampus over time. There are more stem cells when we are young and fewer as we age. This view is in contrast to that of earlier neuroanatomists who considered the nervous system as fixed and incapable of regeneration. The new concept of adult neurogenesis is an example of a long-held scientific theory being overturned.
The hippocampus is virtually unique in its ability to grow new neurons in the adult brain. The challenge is to find a drug that can enhance the process. Neuralstem’s technology uniquely allows the Company to grow (in vitro) regionally specific neural cells of the adult human brain in basically unlimited quantities. Neuralstem was founded as a neural stem cell therapy company, but its technology provides a unique technology window in which to observe important biology in the brain through screening potential drug candidates against its unique cell lines.
The discovery of NSI-189 started with a contract awarded from the Defense Advanced Research Projects Agency (DARPA) to screen for and discover a new class of drugs to specifically protect and enhance hippocampal neural stem cells in the treatment of brain injuries. This grant was in conjunction with DARPA’s Warfighter of the Future program. One of Neuralstem’s stem cell lines produces hippocampal neurons that can differentiate in cell cultures. These cells were used to screen for new drug candidates that might protect and repair the hippocampus in the case of brain injury.
Traditional drug development efforts focused on this therapeutic goal must decide on a molecular target in the brain and develop a drug that will interact with the target. This is done without knowing if this will produce a therapeutic effect. Neuralstem could take a different approach by screening drugs against hippocampal cells in a culture dish. They could be agnostic about the precise target and just look for a molecule that could cause neurogenesis.
They were able to test compounds against the cells with all manner of toxic insults applied, which can be thought of as models of various diseases like stroke for instance; and then test compounds against the various stages of neurogenesis. The Company found that it could influence neurogenesis using different compounds that worked at different phases in the process.
This was the basis for world’s first (and I think the only) thorough search for a new class of drugs that were specifically neurogenic in the human hippocampus, based on screening against actual human hippocampal neural stem cells. As the Company began this process, more and more evidence began to accumulate showing a very high correlation between hippocampal atrophy and depression. This encouraged Neuralstem to look at depression as a first indication for NSI-189.
NSI-189 has a dramatically different mode of action from any drugs now used to treat depression. Neuralstem has conducted tests in cell cultures and in animals which indicate that NSI-189 re-stimulates the growth of neurons in the hippocampus. Research on the current crop of central nervous system drugs has been largely based on the manipulation of neurotransmitters such as serotonin and dopamine. While this approach has led to the creation of drugs like Prozac and Zoloft that produced tens of billions of dollars of sales, the lack of anything really new in this area for the last two decades suggests that this research course has largely exhausted itself.
CNS drug research has been so unproductive that many big pharmas have simply opted out of the space. This is despite the significant unmet medical need resulting from current drugs having only mediocre efficacy. NSI-189 could represent a paradigm shift from the traditional small molecule drugs for depression that primarily target serotonin and other neural transmitter levels in the brain. Neuralstem believes that NSI-189 is reversing hippocampal atrophy and most likely increasing neurogenesis in the hippocampus.
Evidence is mounting that hippocampal atrophy is implicated in all manner of psychiatric and cognitive indications, Consequently, Neuralstem or potentially partners could be developing other small molecule drugs to treat neurodegenerative, cognitive as well as neuropsychiatric, disorders. The Company is already exploring things like stroke, traumatic brain injury and Alzheimers in pre-clinical collaborations. But that is a story for another time and this report focuses on NSI-189 for depression.
Preclinical data suggested that NSI-189 significantly stimulated the generation of new neurons (neurogenesis) in vitro and in animal models. In mice, NSI-189 stimulated neurogenesis of the hippocampus and increased its volume on the order of 20%. NSI-189 also stimulated neurogenesis of human hippocampus derived neural stem cells in vitro. This supported the hypothesis that NSI-189 may reverse the human hippocampal atrophy seen in major depression and other central nervous system disorders.
NSI-189 is beleived to structurally rebuild the hippocampus, inducing up to a 20% increase in hippocampal volume if results in animal models are replicated in humans. Neuralstem attributes this to neurogenesis. This is the world’s first truly neurogenic drug that is designed to specifically increase the number of synapses in the hippocampus. But as with all neuroscience, the story is complicated. While investigators believe that they definitely are seeing neural genesis, it may well be that there are also overlapping pathways providing benefit.
The Positives and Negatives on NSI-189
NSI-189 is a very exciting drug concept and the phase 1b results have given an important signal of efficacy. It is easy to get carried away and start dreaming of a new approach to treating depression that could result in a several billion drug like Prozac or Zoloft. It is important to temper enthusiasm by carefully considering the negatives or uncertainties as well as the positives. Hence, I am starting this section by first going through some of the negatives or uncertainties.
Negatives
This is an entirely new and unprecedented drug approach. We have no experience with a drug that grows new neurons in the brain. What are the long term effects of this? Could we cause too much growth that could actually be damaging? What might unintended consequences be in other parts of the brain? Is the result really coming from neurogenesis or from some other effect?
How should the drug be dosed? If it is growing new neurons, it doesn’t seem as if we would just want to keep on doing this forever. Is there a point at which the therapy must be stopped? Can we then restart the therapy at some future point? Can the process of neuron regeneration be stopped by halting the drug or have we put in process something that continues long after drug therapy is stopped. If so, what are the implications for safety and efficacy?
The phase 1b study was a first in human trial for the drug. Only 18 patients have been treated with NSI-189. This is an extremely small number of patients from which to derive conclusions on what could happen when it is used on hundreds and then thousands of patients. The trial was done under carefully controlled conditions in which the patients were hospitalized for 28 days and carefully watched and medicated. What happens when the drug is used in the community setting? Is 28 days the right amount of time to dose the drug? Most current anti-depressants are used for a half year to a year.
The disease target of depression has very different characteristics than Neuralstem’s NSI-566 which is being used to treat ALS; the latter is a life threatening disease with no effective therapy. Depression can also be life threatening but not to the extent of ALS and there are effective, if not optimal, therapies available. In the case of ALS, uncertainties about long term efficacy and side effects are not of as much of a concern as they will be in depression. Much more complicated, lengthy and expensive clinical trials will be needed to determine the efficacy and side effects of NSI-189 in depression than in NSI-566 in ALS.
The next step in clinical development will be a phase 2 trial that should start in the second half of this year. Neuralstem had thought about partnering the drug but now has sufficient funds to conduct the trial itself. This trial will probably take 18 to 24 months to complete and could cost between $5 million and $10 million dollars. The trial could complete in mid-2016 and between now and then there is not likely to be a lot of news flow.
The phase 3 trials could start in early 2017 and might take 2 to 3 years to complete so that we might see data in 2019 or 2020. The regulators will require very large trials involving thousands of patients. The cost of conducting these trials will be several hundreds of millions of dollars.
This is not a comprehensive list of the negatives.
Positives
In a small phase 1 b trial like this, the primary goal was to determine safety. The number of patents was too small to expect more than a hint at efficacy. Surprisingly, on two of the four measures of efficacy used in the trial, two reached very strong statistical significance, one was close to statistical significance while the fourth was negative. There seems to be a very strong and unexpected signal of a therapeutic effect.
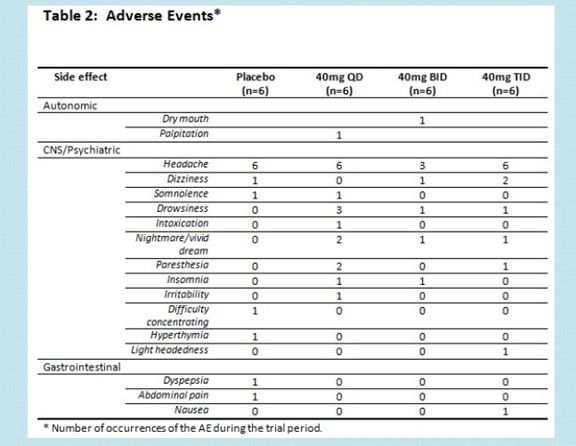
The side effect profile over the 28 days of treatment and through 56 days of follow-up (84 days in total) looked similar to placebo.
Depression has a very high incidence of occurrence. Some of the current drugs used to treat depression recorded sales of $3 to $4 billion before their patents expired. Currently used drugs are less than optimal and there is a great unmet need for effective new drugs. A drug with a different mechanism of action that is effective would have sales potential of $5 to $10 billion or more in the 2025 period.
Drug research in depression has hit a brick wall and many of the large firms have given up development efforts. There is no company that I am aware of that has a drug anywhere comparable to NSI-189. While the clinical development pathway is long, Neuralstem has a clear first mover advantage. Also, companies wanting to compete may not have the technology needed to compete. Unlike many other drug programs in which there is intense competition, Neuralstem is the clear leader with this approach and may not be challenged for many years.
Upcoming Events
Additional data from the NSI-189 Phase Ib trial will be presented at the International College of Neuropyschopharmacology (CINP) 2014 World Congress, in Vancouver, Canada. A poster entitled, "Effects of NSI-189, a neurogenic compound, on quantitative electroencephalography (qEEG) in patients with major depressive disorder (MDD) during a phase 1b randomized, double-blinded, placebo controlled, multiple ascending dose study," will be presented in the session "Biomarkers (incl. pharmacogenomics and brain imaging) for diagnosis and treatment response" on June 24th from 5:15 to 6:45 p.m. PDT.
During the course of the NSI-189/MDD Phase Ib trial, patients underwent quantitative electroencephalography brain measurements, in addition to the clinical tests discussed in this report. In the study to be presented at CINP
(https://www1.cinp-congress.org/guest/ID30b935a2860cf8/AbstractView?ABSID=11795)
Researchers reported that NSI-189 had a measurable impact on the brain waves of patients on active therapy who showed increased high-frequency brain wave activity as compared to placebo in the left temporal region, where the hippocampus is located. This finding is consistent with improvement in left temporal lobe function and may also reflect changes in activity in the left mesial temporal lobe and hippocampus, and changes in patient clinical responses.
These findings substantiate the hypothesis demonstrated by studies in cell cultures and animal studies that NSI-189 stimulates the neurogenesis of hippocampal stem cells. EEG findings show that the hippocampal region is where a significant change in brain wave activity is taking place in patients on active therapy, and corroborates the theory that NSI-189 targets the hippocampus. It is the first physical proof in humans. Taken together with the significant patient clinical improvements reported last week, this suggests that NSI-189 may be affecting the physical structure of the brain in patients with depression.
Background on Major Depressive Disorder and Its Treatment
Before discussing the results of the phase 1b trial of NSI-189 in major depressive disorder (MDD), I thought I would review the disease, how it is treated and how the effectiveness of drugs used to treat MDD is measured. Prozac was introduced in 1987 and ushered in a new generation anti-depressant drugs using similar mechanisms of action. Determined efforts by pharmaceutical firms to find other effective drugs having different mechanisms of action have been frustrating; this has led to a de-emphasis of research in this area.
Major depressive disorder (MDD) is also referred to as clinical depression, major depression, unipolar depression or unipolar disorder. The hallmark symptoms of the disease are persistent moodiness, loss of self-esteem and loss of interest in the pleasures of daily life. It is a disabling disease that affects not only the patients but their families and friends and workplace performance.
The cause of depression can only be guessed at. It has been variously attributed to social pressures, genetics and biological factors. Most biological theories focus on imbalances in the monoamine chemicals serotonin, norepinephrine and dopamine, which are naturally present in the brain and assist in communication between nerve cells. The anti-depressant drugs work by modulating the effects of one or more of these neurotransmitters.
It is estimated that 1 in 10 adults suffer from some form of depression. The most common time of onset is between the ages of 20 and 30 years, with a later peak between 30 and 40 years. It is further estimated that 3.4% of patients with MDD commit suicide and up to 60% of people who commit suicide had depression or another mood disorder.
There is no laboratory test that can be used to directly diagnose MDD and allow planning for treatment as in the case for drugs that lower cholesterol or blood pressure or that treat cancer or infectious disease. The diagnosis is based on an evaluation of how the patient is functioning using scales that are based on questions asked of the patient by trained physicians. The questions are weighted to give a quantitative measure of the illness. The subjectivity of these scales adds somewhat more uncertainty in analyzing the effect of treatments for MDD, but this is all we have.
Treatment of MDD
There are three common treatments for major depressive disorder: psychotherapy, anti-depressant drugs and electroconvulsive therapy.
Antidepressant Drugs (Primarily SSRIs)
There are several classes of drugs used to treat depression but selective serotonin reuptake inhibitors (SSRIs) account for most usage. Anti-depressants are one of the most frequently prescribed classes of drugs with over 30 million prescriptions written per year. Several SSRIs reached blockbuster sales levels. Before going off patent Eli Lilly’s Prozac, Pfizer’s Zoloft and Forest Laboratories Lexapro respectively reached peak sales of $4.0 billion, $3.5 billion and $2.5 billion. At their peak, sales of anti-depressants were about $15 billion, but as is the case Prozac, Zoloft and Lexapro most of the drugs in this class are now generic. There has been little innovation in MDD drugs and many big pharma companies have given up on research in this area
Selective serotonin reuptake inhibitors (SSRIs) like Prozac, Zoloft and Lexapro are the primary medications prescribed, owing to their relatively mild side-effects, and because they are less toxic in overdose than other antidepressants. Patient response to anti-depressants is variable. It is estimated that the first drug given is only effective in 50% to 75% of the cases. Patients who do not respond to one SSRI can be switched to another antidepressant, and this results in improvement in almost 50% of cases.
It usually takes six to eight weeks from the start of treatment to begin to see an effect from a drug. Treatments are given until remission occurs and then continued for perhaps 16 to 20 weeks to minimize the chances of recurrence. People with chronic depression may need to take medication indefinitely to avoid relapse but most patients are on therapy for less than one year.
For children, adolescents, and young adults between 18 and 24 years old; there is a higher risk of both suicidal ideation and suicidal behavior when treated with SSRIs. A black box warning was added to product labels in the United States in 2007 on SSRIs and other antidepressant medications due to increased risk of suicide in patients younger than 24 years old. For adults, it is unclear whether or not SSRIs affect the risk of suicidality.
Psychotherapy
Psychotherapy is usually given on an outpatient basis on a one on one basis. It can be delivered by mental health professionals, including psychotherapists, psychiatrists, psychologists, clinical social workers, counselors, and suitably trained psychiatric nurses. The effects of antidepressants are generally beleived to be somewhat superior to psychotherapy, especially in cases of chronic major depression. It is more applicable in milder forms of depression and patients tend to prefer it over drugs, probably because they don’t have to deal with the side effects.
Electroconvulsive therapy
Electroconvulsive therapy (ECT) is a procedure whereby pulses of electricity are sent through the brain via two electrodes that are placed on the temples with the patient under a general anesthetic. A shock is delivered that induces a seizure. Because of the physical and social trauma of ECT, it is usually reserved for severe depression that has not responded to drug therapy.
ECT can have a quicker effect than antidepressant therapy and thus may be the treatment of choice in emergencies such as catatonic depression where the person has stopped eating and drinking or where a person is severely suicidal. It is probably more effective than pharmacotherapy for depression in the immediate short-term. However, the relapse rate in the following six months is high at an estimated 50% or more
Evaluating the Effect of Therapy on Depression
As was previously mentioned, there is no laboratory test to directly diagnose MDD and allow planning for treatment as for example in the case for drugs that lower cholesterol or blood pressure or that treat cancer or infectious disease. This means that the assessment of the effect of treatment on depression is not as straight forward as with those diseases. There is no biological marker to verify that therapy-whether psychotherapy, drugs or electroconvulsive therapy- is having the desired effect.
Investigators have come up with other techniques for determining whether depression therapies are effective. They consider the symptoms of the disease and come up with a number of questions that address those symptoms. A number scale is assigned to each symptom based on the subjective judgment of a physician. There are a number of scales that have been designed. One of those used in the phase 1b trial was the Montgomery-Asberg Depression Rating Scale (MADRS). I describe it below to give you an idea as to how scales are constructed:
Montgomery-Asberg Depression Rating Scale (MADRS)
MADRS is based on a clinical interview with the patient by a trained physician in which a number of questions are asked about symptoms. The rater then must then make a subjective evaluation in which the severity of each symptom is rated on a scale from 0 to 6; the higher the MADRS rating the more severe the symptom.
There are ten symptoms that are addressed by a series of questions. A higher MADRS score indicates more severe depression, and each item yields a score of 0 to 6. The overall score ranges from 0 to 60. The questionnaire includes questions on the following symptoms:
- Apparent Sadness Representing despondency, gloom and despair, (more than just ordinary transient low spirits) reflected in speech, facial expression, and posture. Rated on depth and inability to brighten up.
- Reported Sadness Representing reports of depressed mood, regardless of whether it is reflected in appearance or not. Includes low spirits, despondency or feeling of being beyond help without hope. Rated according to intensity, duration and the extent to which the mood is reported to be influenced by events.
- Inner Tension Representing feelings of ill-defined discomfort, edginess, inner turmoil mounting to either panic, dread or anguish. Rated according to intensity, frequency, duration and the extent of reassurance called for.
- Reduced Sleep Representing the experience of reduced duration or depth of sleep compared to the subject’s own normal pattern when well.
- Reduced Appetite Representing the feeling of loss of appetite compared with when well. Rate by loss of desire for food or the need to force oneself to eat.
- Concentration Difficulties Representing difficulties in collecting one’s thoughts mounting to incapacitating lack of concentration. Rate according to intensity, frequency, and degree of incapacity produced.
- Lassitude Representing a difficulty getting started or slowness initiating and performing everyday activities.
- Inability to Feel Representing the subjective experience of reduced interest in the surroundings, or activities that normally give pleasure. The ability to react with adequate emotion to circumstances or people is reduced.
- Pessimistic Thoughts Representing thoughts of guilt, inferiority, self-reproach, sinfulness, remorse and ruin.
- Suicidal Thoughts Representing the feeling that life is not worth living, that a natural death would be welcome, suicidal thoughts, and the preparations for suicide. Suicidal attempts should not in themselves influence the rating.
The usual cutoff points for MADRS in measuring the severity of depression are;
- 0 to 6 – normal/symptoms absent
- 7 to 19 – mild depression
- 20 to 34 – moderate depression
- >34 – severe depression.
Other Scales Used in the Phase 1b Study
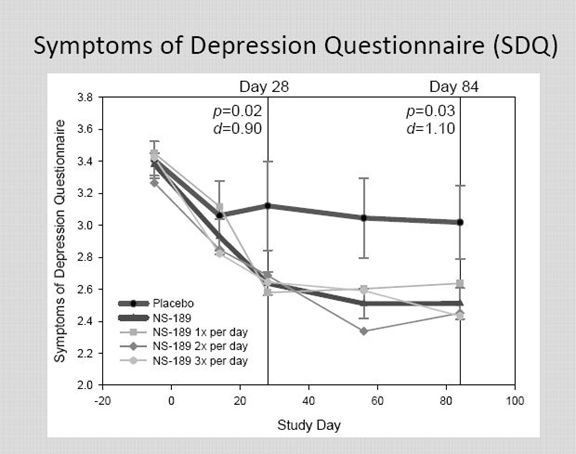
Montgomery-Asberg Depression Rating Scale (MADRS) is a very widely used and validated scale. In addition to MADRS, the results were also judged on three other scales: Clinician Global Impression – Improvement (CGI-I), Symptoms of Depression Questionnaire (SDQ) and the Cognitive and Physical Functioning Questionnaire (CPFQ). I am not going to discuss these in detail, but the idea behind each is to assign a numerical value to subjective judgments made by a rater about the severity of the depression using a set format.
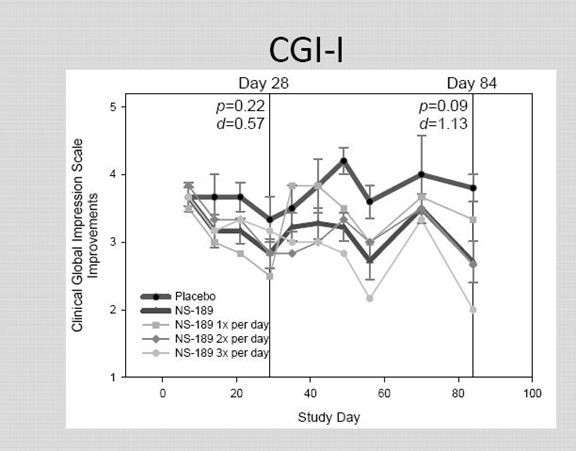
The Clinical Global Impression - Improvement scale (CGI-I) is a 7 point scale that requires the clinician to assess how much the patient's illness has improved or worsened relative to a baseline state at the beginning of the intervention and rated as: 1, very much improved; 2, much improved; 3, minimally improved; 4, no change; 5, minimally worse; 6, much worse; or 7, very much worse. Many researchers, while recognizing the validity of the scale, consider it to be subjective as it requires the user of the scale to compare the subjects to typical patients in the clinician experience.
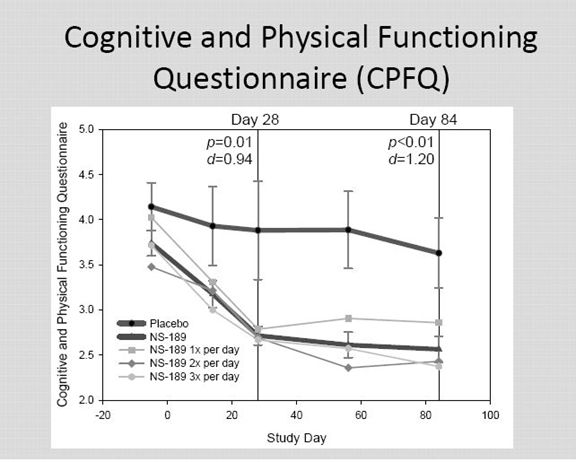
The Cognitive and Physical Functioning Questionnaire (CPFQ) is a scale like MADRS that was developed by the Harvard Medical School and Massachusetts General Hospital.
Statistical Analysis of the Study
The results of the study were statistically analyzed using the well-known null hypothesis technique. This starts with the assumption that results in the placebo group and the drug group are the same and tries to disprove that assumption. It then tests this hypothesis by calculating a p-value which can be anywhere from very small to very large. A p-value of 0.05 means that there is only a 5% or 5 out of 100 chance that the drug and placebo have the same effect. This is the cutoff point for judging statistical significance in drug trials.
The 0.05 is an arbitrary number but there has to be some cutoff and this has been broadly accepted. Hence, a clinical trial in which p≤ 0.05 is judged a success and p>0.05 is judge a failure. However, the p value is very much influenced by the number of patients in a trial. In this particular phase 1b trial, there were only 18 patients treated with the drug and 6 with placebo. The phase 2 trial being planned will probably involve 200 to 300 patients and the ultimate phase 3 trials could involve several thousand. In such a small trial as this, investigators generally do not expect a p≤ 0.05; they are looking for a numerical trend that is a signal of efficacy. If the signal is a true one, the p values in the larger phase 2 and 3 trials would be much less.
Most investors are familiar with the p value and understand its importance. However, in this trial the investigators also reported the Cohen’s d factor. This is less well understood and really takes more statistical expertise than I have to fully appreciate and explain, but I will give it a try. The Cohen d compares the placebo group and the drug group. It measure whether the means of the two groups are shifted by measuring how many standard deviations the mean has shifted. This shift can be negative or positive but is always expressed as a positive.
In order to calculate the Cohen d you need to know the means and the standard deviations of the placebo and drug groups. The numerator used to calculate the Cohen d is the mean of the control group minus the mean of the experimental group. The denominator is then determined by first adding the standard deviation of the control and the standard deviation of the drug group; this sum is then divided by two.
I am not going to try to go much further into this measurement. It is a descriptive statistic that conveys the estimated magnitude of a relationship between the placebo and drug group without making any statement about whether the apparent relationship in the data reflects a true relationship in the population. In this way, statisticians feel that it complements inferential statistics such as p-values. A really small effect is considered to be d <0.20, a moderate effect is d =0.2 to 0.5 and a large effect is anything greater than 0.50. Investors should look for a high Cohen d in clinical trials, i.e. >0.50.
In looking at small data samples in which the p value is much less precise (as in this trial), the Cohen d can give some added comfort as to the potential validity of the p value. Because we are looking at a control group of 6 placebo patients and three groups of 6 patients each given a different dose of the drug, the Cohen d factor helps in the evaluation of the data.
A controversial meta-analysis done in 1998 performed on all of the studies of anti-depressant drugs. The study showed that for all of the widely used anti-depressant drugs that the Cohen d value was somewhere between 0.2 and 0.5 indicating a moderate effect not markedly different from placebo. Keep this in mind as the Cohen d in the NSI-189 trial was much larger as the Cohen d factors were generally very strong.
Analyzing the Results of the NSI-189 Phase 1b Trial
The abstract on the phase 1b trial of NSI-189 was presented at the American Society of Clinical Psychopharmacology Annual Meeting on June 17th in an oral presentation. The title was "A Phase Ib Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose Escalation Study Evaluating the Effects of NSI-189 Phosphate, A Neurogenic Compound, in Patients with Major Depressive Disorder (MDD)."
The Abstract
The abstract for the study is presented below:
"A Phase Ib Randomized, Double-Blind, Placebo-Controlled, Multiple-Dose Escalation Study Evaluating the Effects of NSI-189 Phosphate, A Neurogenic Compound, in Patients with Major Depressive Disorder (MDD)"
Authors: Maurizio Fava1, Karl Johe, Lev Gertsik, Larry Ereshefsky, Bettina Hoeppner, Martina Flynn, David Mischoulon, Gustavo Kinrys, Marlene Freeman
Institutions involved: Massachusetts General Hospital Department of Psychiatry, Neuralstem, Inc., California Clinical Trials Medical Group, PAREXEL International, Harvard Medical School,, Massachusetts General Hospital Clinical Trials Network and Institute, Massachusetts General Hospital, Harvard Medical School, Massachusetts General Hospital
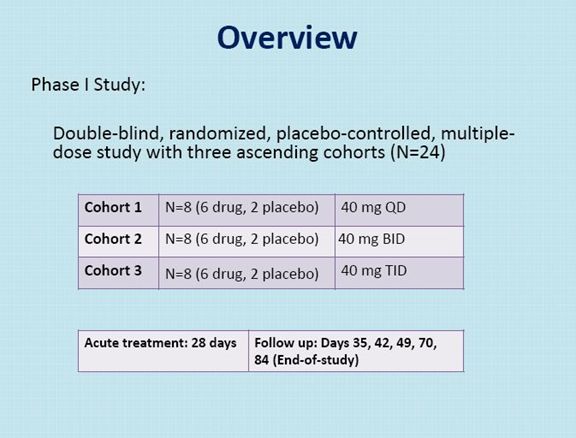
NSI-189, a benzylpiperizine-aminiopyridine, is a novel chemical developed by Neuralstem Inc. for the treatment of major depressive disorder, based on preclinical evidence of neurogenesis in human hippocampus-derived neural stem cells in vitro and in mouse hippocampus in vivo. NSI-189 has also shown behavioral efficacy in the novelty suppressed feeding after daily oral administration for 28 days. This is an early phase, double-blind, randomized, placebo-controlled, multiple-dose study with three ascending cohorts.
The first cohort received 40 mg QD, the second cohort 40 mg BID, and the third cohort 40 mg TID. 24 patients with major depressive disorder (MDD) were recruited, with their diagnosis and illness severity confirmed through an independent, remote SAFER interview from the MGH CTNI raters. Each cohort included at least 3 female subjects. Each patient underwent a Screening for eligibility (Day -37 to Day -6 or -3) and eligible patients were admitted into the unit on Day -5 to complete their wash-out and be reconfirmed for eligibility and for baseline assessments. Eligible patients receive daily dosing of investigational medicinal product (NSI-189 Phosphate or placebo) for 28 days starting on Day 1 and were followed for safety, PK, and PD until discharge. At the conclusion of in-house dosing (Day 28), patients remained in the unit for up to 3 additional days, at the psychiatrist’s discretion.
On Day 35 (± 3), Day 42 (± 3), Day 49 (± 3) and Day 70 (± 3) outpatient follow-up visits took place. Patients returned to the unit for extensive follow-up on Day 56 (± 3) and Day 84 (± 3) (End-of-study). The efficacy assessments included the Montgomery-Asberg Depression Rating Scale (MADRS), the Clinician Global Impression – Improvement (CGI-I), the Symptoms of Depression Questionnaire (SDQ) and the Cognitive and Physical Functioning Questionnaire (CPFQ).
(Author’s note: The following is the most important paragraph)
Despite the minimal improvement observed among the placebo-treated patients, at day 28, the efficacy measurements showed a clinically meaningful reduction in depressive and cognitive symptoms across all measures for the two lower doses (40 mg/day and 80 mg/day) but not for the highest dose (120 mg/day). These improvements appeared to persist over time during the follow-up for MADRS, SDQ, and CPFQ. In terms of safety, no serious AEs occurred and the drug was well tolerated. The main limitations of this study are the relatively small sample size of each cohort and the fact that efficacy analyses were not the primary aim, and were meant to be only descriptive in nature.
In summary, a novel neurogenic compound, NSI-189, has shown promise as a potential treatment for MDD in a Phase 1B, double-blind, randomized, placebo controlled, multiple-dose study with three ascending cohorts. These preliminary findings support the view that a neurogenesis-based platform can identify promising new treatments for MDD (Fava et al, 2012). The possible inverted U dose-response curve observed in this study is consistent with previously reported inverted U dose-response curve of compounds enhancing synaptic plasticity (Laverne and Jay, 2010). Further studies are necessary to confirm these preliminary observations.
Learning Objectives:
- To learn about the potential usefulness of novel neurogenic compounds in depression.
- To learn about the safety and efficacy of NS189 in the treatment of depression
Interpretation of Slides Used In Presentation
The oral presentation went into much greater detail than the abstract and had a slide presentation. I am enclosing some of the key slides used in this presentation along with some of my comments on each.
There were a total of 18 patients on drug and 6 on placebo.
For the first 28 days of the study patients were kept in a hospital. This was because NSI-189 has a unique mechanism of action in that it may grow new neurons. This created a concern about safety.
Active drug treatment was given for 28 days and then patients were followed through day 84. This length of time is less than that for currently used anti-depressant drugs which are are generally used for one-half year to a year.
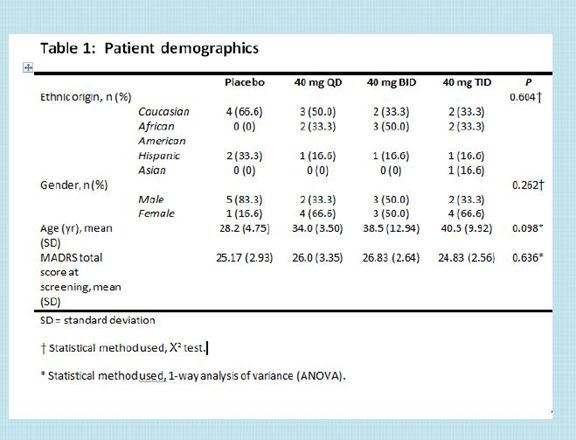
Patients entering the study could be male or female and 18 to 60 years old who were diagnosed with recurrent depression.
They could be taking an anti-depressant (s).
They had to have had at least two prior depressive episodes with one being current.
The mean MADRS scores at the time of entry in the study were 25 to 27 indicating they were moderately depressed; moderate depression is generally defined as 20 to 34 on the MADRS scale.
The side effects were generally comparable to placebo over this 28 day period.
There is a hint of more drowsiness or paresthesia with NSI-189.
Paresthesia is a sensation of tingling, tickling, prickling, pricking, or burning of a person's skin with no apparent long-term physical effect.
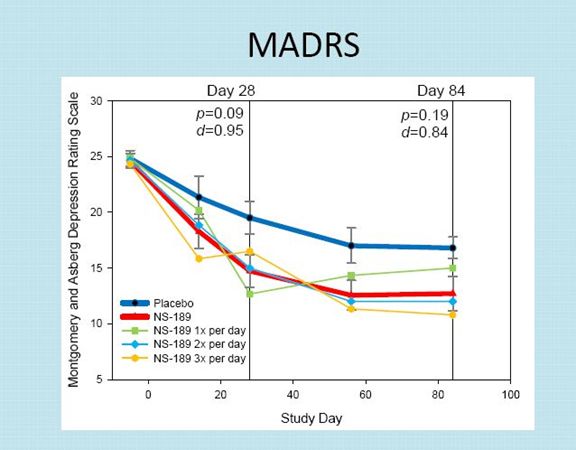
The MADRS results indicate that there was a placebo effect at day 28 that caused about a 5.0 point drop in the MADRS scale.
The drop in the MADRS scale for NSI-189 was about 10 points averaged across the three dosing groups at the end of 28 days. KOLS consider this as quite clinically significant.
There was a sustained effect for both the placebo group and the drug group at day 84. Interestingly, the placebo group and drug group showed an improvement from day 28 to 84. This is curious, but depression trials are known for producing curious results.
At the end of day 84, which is 56 days after the last drug treatment, there continued to be a sustained effect on MADRS. At day 84, there was about a 4 point drop in the MADRS score for NSI-189 versus placebo.
Based on this limited sample, the dosages of NSI-189 given twice a day and three times a day seemed to have about the same effect and both appeared to be better than once a day, but these are small numbers.
NSI-189 did not reach statistical significance based on p value at days 28 or 84 although this is not unusual or unexpected in a small sample size. The trend looked encouraging and the Cohen d was very strong at 0.95 ay day 28 and 0.84 at day 84.
This is a scale comparable to MADRS that was developed by Harvard and Massachusetts General. It use seven items rather than the ten used by MADRS
Here the p values and Cohen d values were very positive at both day 28 and day 84.
I am unable at this point to discuss the difference in MADRS and CPFQ and whether more weight should be put on one or the other. MADRS has been in use for much longer.
Considering the small numbers, the very strong p values and Cohen d values for CPFQ and the encouraging p values and Cohen d values from MADRS; I think that the scales reinforce each other.
This is a 43 question scale with which I am less familiar than MADRS. I need to do some work on the relative importance of this scale.
Obviously, the p values and Cohen d values are very impressive at both days 28 and 84.
The CGI-I results are unimpressive.
I think that this scale probably has less value than the other three efficacy scales. It is felt that it requires even more subjectivity on the part of physicians.
Tagged as CUR, Inc., major depressive disorder, neural stem cells, Neuralstem, Inc., NSI-189 + Categorized as Company Reports
6 Comments
Trackbacks & Pingbacks
-
Neuralstem: Previewing Upcoming Important Events and Some Thoughts on the StemCell Patent Infringement Lawsuit (CUR, $3.62 Buy, August 26, 2014) | Expert Financial Analysis and Reporting | Smith on Stocks
[…] reports. For those that would like a refresher on NSI-189, I would recommend that you refer to : Phase 1b Results for NSI-189 are Very Encouraging but It Is Early Days and An In-Depth Look at NSI-189, A Novel Small Molecule Drug Being Studied in Major Depressive […]
-
Notes from Neuralstem Presentation at Biotech Showcase Conference, January 13, 2015 (CUR, Buy, $3.09) | Expert Financial Analysis and Reporting | Smith on Stocks
[…] The trial of the small molecule drug NSI-189 in major depressive disorder will start in April. It should be about 150 patients in 20 centers. It is expected to take 18 to 24 months to complete. This product will be partnered after the results of the phase 2 trial are known assuming success in the trial. Mr. Gar discussed the positive phase 1 results for NSI-189. I analyzed them in a report last June if you are interested. […]
Comment
You must be logged in, or you must subscribe to post a comment.





This is new, right? I think it’s a smart move; they’ll get more $$$ to get a partner for Phase 3 rather than for Phase 2… and I doubt they can afford Phase 3 on their own.
“…Neuralstem had thought about partnering the drug but now has sufficient funds to conduct the trial itself. This trial will probably take 18 to 24 months to complete and could cost between $5 million and $10 million dollars. The trial could complete in mid-2016 and between now and then there is not likely to be a lot of news flow.
The phase 3 trials could start in early 2017 and might take 2 to 3 years to complete so that we might see data in 2019 or 2020. The regulators will require very large trials involving thousands of patients. The cost of conducting these trials will be several hundreds of millions of dollars…”
I’m curious about the choice of delivery systems. Is there a reason the drug is being administered orally instead of by direct, on-site injection? Would that have even been possible with the hippocampus? Or is the whole point to find a treatment that is non-intrusive as well as successful?
Direct injection would be pretty invasive and much less acceptable to patients; it’s preferable to take a pill than even to get IV meds, let alone any type of administration that gets directly into the central nervous system. If successful NSI-189 could be truly revolutionary, especially if the treatment effect lasted beyond the duration of administration.
It would not be pratical to inject stem cells into the hippocampus to treat depression. Bear in mind that NSI-189 is a small molecule drug. It is not a stem cell product like NSI-566. NSI-189 is a very unique drug.