Neuralstem: My Analysis of Phase 1 and Phase 2 Results for NSI-566 Neural Stem Cells in ALS Indicates Encouraging Signals of Efficacy (CUR, Buy, $1.25)
Putting This Report in Perspective
This is an extensive report that goes over the aggregate results of the phase 1 and 2 results for Neuralstem’s nSI-566 neural stem cells in ALS. I have also reported on the observations of Dr. Eva Feldman who is the principal investigator in these trials and is hopeful and optimistic for success in an upcoming phase 2b/3 trial. She has been involved for the last ten years with the development of these cells from the pre-clinical stage through human clinical trials. Her experience has afforded some valuable insights for me that I am sharing with you.
The use of neural stem cells to treat ALS and other neurological diseases is a potential paradigm shift in treating these diseases which have been unresponsive to traditional pharmaceutical product development strategies. I see the use of neural stem cells as having the potential to have an impact on drug development of the same impact as recombinant DNA and monoclonal antibodies. However, development is in a very early stage and has not yet achieved proof of concept although there are intriguing signals of therapeutic efficacy in ALS, a rapidly progressing, almost always fatal disease in which no new drugs have been approved in 20 years.
Investors must put in perspective that drug development with a new technology base like stem cells can take several decades to reach mainstream investor or pharmaceutical industry acceptance. Neuralstem is at the very forefront of stem cell development, but is a small company with limited resources. It is amazing how far they have progressed in the last decade, but the challenge they face is enormous. I believe that the results seen so far in ALS are encouraging. In a meaningful percentage of ALS patients, we have seen a stabilization of ALS for as long as one to four plus years. ALS is an inexorably progressing disease in which the vast majority of patients die in about two to three years after diagnosis. Stabilization of ALS in any patient is highly unusual.
While there is reason for hope and excitement, there are numerous issues to be concerned about. Critically, only 27 patients have received these stem cells so far. Of these, perhaps only 20 might be expected to benefit from treatment. It may take treatment in hundreds or thousands of patients to more accurately define the therapeutic potential and limitations of this therapy and refinement to approved product status could take years or decades. I view this investment as an asymmetric opportunity which has incredible upside potential, but also the risk that obstacles could arise that could result in investors losing all or almost all of their investment. This investment should only be considered by investors willing to take that risk, like me.
Investors must be willing to delve deeply into what is known and what is unknown about this potentially paradigm changing technology. I would urge investors to take the time to read my full report.
My Key Observations:
Phase 1 results are extremely impressive: By my analysis, phase 1 results provide a clear signal of activity of Neuralstem’s NSI-566 neural stem cells in the treatment of ALS. In the phase 1 trial of 12 patients, I believe that it is correct to exclude the first six patients treated from an efficacy analysis. These were non-ambulatory (paraplegic) patients whose inclusion in the trial was solely to determine if the cells could be safely implanted. I have also excluded another patient who died of a heart attack not attributable to ALS in the opinion of the investigator; this leaves 5 evaluable patients. Notably, each of the 5 experienced dramatic efficacy showing in some cases stability in ALS for up to 4 ½ years as determined by the ALSFRS-r scale. ALS is a disease in which most patients experience an inexorable steady decline and die within two and one-half to three years of diagnosis. Stabilization of ALS in any patient is highly unusual.
Phase 2 results are encouraging also but not nearly as much as phase 1: The phase 2 results have not shown the same dramatic benefit thus far and this has alarmed investors and given rise to significant shorting attack against of the stock. In the phase 2 trial, 15 ambulatory patients were treated. One of these patients, experiences a serious adverse event probably related to surgical technique that makes evaluation of efficacy difficult. Of the other 14 patients, 3 seem to be showing stability in ALSFRS-r similar to the phase 1 results. Seven patients appear to be doing as well or better than what has been seen historically seen on ALSFRS-r with the placebo treated patients and six appear to be doing worse than would be expected. The reason for the latter remains unexplained and is an obvious source of concern.
In regard to the phase 2 trial interpretation of data, I would make several points. The ALSFRS-r scale is a qualitative measure of different items that affect physical functioning and quality of life issues for ALS patients. (There are details on the ALSFRS-r scale in the appendix of this report). Neuralstem has been extremely transparent in showing the ALSFRS-r scores before treatment has begun, at baseline and for the length of time from surgery for each ALS patient. In looking at the data, it looks like the scores can jump around over periods of months. The results for patients in phase 2 are based on 100 to 490 days of observation as opposed to 640 to 840 days for the phase 1 patients. As the phase 2 patients are followed for longer periods of time, the assessment of change in ALSFRS-r status could change (for better or worse.)
Looking at combined phase 1 and 2 results: Here is my preliminary assessment of the results of the phase 1 and 2 trials. The phase 1 results in the 5 patients is extraordinary. I think that this gives is a clear signal of activity. The results in phase 2 are much less positive as it appears that 3 of 14 evaluable patients (21%) or 3 of 15 (20%) appear to be achieving a roughly similar ALSFRS-r outcome as seen with the five phase 1 patients. If I combine phase 1 and phase 2 patients, 8 of 19 patients (42%) or 8 of 20 (40%) appear to be achieving a durable stabilization of their disease over a period of 292 to 840 days. Remember that these patients remain alive and as time passes, more meaningful interpretations may be possible.
How do we put these numbers or percentages in perspective? Let me propose an analogy with treatment of metastatic cancer which like ALS rapidly and inexorably worsens and results in almost all patients dying in a one, two to three year period. In some cancers such as non-small cell lung and melanoma, the checkpoint inhibitors (Bristol-Myers Opdivo and Merck’s Keytruda) appear to be achieving durable control of the cancer in about 20% to 25% of cases. These drugs have been hailed as breakthroughs by the FDA and other regulators even though 75% to 80% of patients don’t respond meaningfully.
If you accept this analogy, the phase 2 results for NSI-566 stem cells are as good in ALS as the checkpoint inhibitors in metastatic cancer as they show a durable response of 20+% as compared to 20+%. If we lump together both phase 1 and 2 results, the durable response for NSI-566 stem cells is an extremely impressive 40% to 42%. The following table summarizes my observations on phase 1 and 2 results. This table is explained in more detail later in this report.
Phase 2 results versus phase 1: The obvious question to ask is why phase 2 results are less impressive than phase 1. My first response is that the phase 1 results are so extraordinary that they would be virtually impossible to replicate. Drawing on the example I cited for the checkpoint inhibitors, if the phase 1 results showed ALSFRS-r stabilization of 21% instead of 100% and this was then followed by phase 2 which on a preliminary basis show a 21% stabilization rate, I think that objective observers would be hailing this as an extremely promising signal of activity. Indeed, the principal physician investigators in the study have expressed this point of view and are quite supportive of a phase 2b/3 trial.
In dealing with such small numbers of patients, I think that investors have to be cautious in accepting the percentages that I have presented. Neither may be representative of the potential outcome in a phase 3 trial. However, I think virtually all objective observers would accept the view that there is strong signal of activity in some patients. It is always the case in cancer that there is enormous heterogeneity. For example, breast cancer (and almost all other types of cancer) can manifest itself in many different genetic forms; one genetic type might respond very well to one type of drug and not at all to another. It may be the case in ALS that some types of ALS will respond much better to neural stem cell therapy than others. The challenge is to identify such a patient sub-population.
Differences in phase 1 and 2 Trials: We have to ask ourselves if there were any major differences in the phase 1 and phase 2 patients. In terms of characteristics, five patients evaluated for efficacy in phase 1 and all patients in phase 2 were ambulatory and reasonable healthy within the context of ALS. One of the major differences that I can see is that all of the patients in the phase 1 trial received initial injections in the lumbar region and 3 of 5 also received cervical injections. In phase 2, the first 12 patients received only cervical injections while the final 3 received both cervical and lumbar injections. Could it be that lumbar injections may be more important in ALSFRS-r scores than the investigators thought? Remember that the lumbar region mainly controls muscles in the arms and legs so that any improvement can be more readily felt by the patient. On the other hand, motor neurons from the cervical region control such functions as respiration for which changes in the short term may be less observable. Just a thought.
Patients in the phase 2 trial received more injections and more cells per injection than those in phase 1. In some cases, this was dramatic. This leaves open the question as to whether more cells could be less effective than fewer cells. Could it also be the case that cervical injections can produce more negative effects than lumbar injections? It is also important to understand that patients in these trials were given immunosuppressant drugs as the transplanted cells were allogeneic. These drugs produce significant side effects which can negatively affect quality of life. The ALSFRS-r scale very importantly takes into account quality of life so it may be the case that the ALSFRS-r score is meaningfully, negatively affected by these side effects. Hence in comparing ALSFRS-r scores of NSI-566 treated patients to historical controls, it is possible that the use of immunosuppressive drugs could produce a negative effect not seen in previous control groups in ALS trials.
A hypothesis as to why phase 2 results were disappointing when compared to phase 1: Let me offer a hypothesis as to why initial phase 2 results disappointing relative to phase 1. By focusing on cervical injections, the phase 2 trial was attempting to show an improvement in respiratory function while the lumbar injections primarily used in phase 1 were more likely to affect limb function. One key opinion leader has suggested that respiratory loss in ALS patients occurs much later in the disease progression than the loss of function in limbs. If this is the case, it might be more difficult for the ALSFRS-r scale to show improvements in the short term in respiratory function (as was the intent of the phase 2 trial) whereas an effect on limb function would be detected earlier by the ALSFRS-r scale (as was the case with the phase 1 trial). It will be important to observe the phase 2 patients over a longer period of time to see if there is a stabilization or lesser rate of decline in ALSFRS-r and more particularly in those segments which relate to respiratory function. Put another way, the lumbar controlled muscle groups are areas which are more sensitive to treatment because in the early stages of disease (these were early stage ambulatory patients) they are more rapidly losing arm and leg function than respiratory function.
Statistical analysis of phase 2 results offered by investigators is not convincing at this time: The investigators used a statistical analysis to interpret combined phase 1 and 2 results in a way that investors (me included) did not find convincing. Because of the lack of a control group, they took ALSFRS-r results from the two largest available databases of placebo patients and asked how results in NSI-566 treated patients compared. This resulted in a data base of about 500 patients. The analysis measured how ALSFRS-r of the “average” patient in this 500 patient data base declined. It then compared this average to results in 20 patients (those who might reasonably benefit) in the phase 1 and 2 trials of NSI-566. At a 95% confidence level, they found that 80% of NSI-566 patients were above the level of this “average patient” control. Frankly, I am not giving much weight to this analysis at this time. I think that the patient numbers are too small and the ALSFRS-r results are too variable in the period of time patients in phase 2 patients have been treated. Over time as the data matures, this statistical analysis technique may become more convincing.
This is very positive for future stem cell research: I think that one of the major positives that is virtually undeniable is that neural stem cells can be safely implanted in the spinal cord and that the cells when implanted are safe. Some of the first stem cell transplants used embryonic stem cells that led to significant problems because of their wild, uncontrolled differentiation. Embryonic cells formed tumors in perhaps 5% of transplants in animal models of ALS and differentiated not only into neural stem cells (as desired), but also bone, hair and tooth cells.
Because of this, the FDA was deeply involved in the conduct of both the phase 1 and 2 trials and mandated that Neuralstem proceed slowly and deliberately. In the phase 1 there was at least a month between surgeries and FDA representatives were present at the surgeries. As time went on, the FDA became more comfortable that the neural stem cells did not present the same risk as embryonic stem cells and allowed the surgical procedures to proceeds more quickly. All of this bodes well for future research using neural stem cells in other neurological diseases such as stroke, Alzheimer’s, Parkinson’s, ischemic etc.
What is the next catalyst? The announcement of the design of the next clinical trial will likely be a phase 2b/3 trial, which the Company has hinted as being a 50 patient trial with a control arm. If the FDA consents to the trial design, it will be a clear indication that the trial is clinically meaningful in the view of regulators and could produce positive results. Seldom is the FDA as involved so closely as in these phase 1 and 2 trials. Allowing the Company to proceed to a phase 2b/3 clearly signals that the FDA views the NSI-566 stem cells as safe, but that there are also clear signals on important clinical activity. The surgical procedure involving transplanting NSI-566 stem cells is a complicated surgical procedure that exposes the patients to some risk of negative complications. FDA would only allow the trial to go forward if it believed that the potential benefit substantially offset the potential risk.
Why aren’t the major biopharma companies doing stem cell research or just acquiring Neuralstem? The use of stem cells to treat neurological diseases is a paradigm shifting technology. The history of the big pharma companies is that they have been slow to become involved in paradigm shifting technologies. For example, monoclonal antibodies for various diseases are now the most extensively studied drugs in the world. This technology was first described in the early 1970s and the first truly important drug was introduced in 1999; this was Idec’s Rituxan. Only in the last five to ten years have major pharma companies jumped into development after skeptically watching the technology develop over 30+ years. Similarly, the checkpoint modulators being developed for immune-oncology are the hottest area of cancer research on the planet and every drug company is scrambling to gain a foothold. Bristol-Myers was the first to recognize the promise and jumped into development by acquiring Medarex in 2009. Even then most companies were skeptical and took a wait and see approach.
The situation with neural stem cells is similar to what we have seen before. The big companies will wait until proof of concept is achieved by small entrepreneurial companies like Neuralstem. At that point, there would be a bidding war for Neuralstem and other stem cell companies like Stem Cells, Inc.
Stem cell development will take time: I view stem cells as having the potential to be a paradigm shift in technology that could be every bit as industry changing as monoclonal antibodies and checkpoint modulators. It is important to understand that this research as in the case of monoclonal antibodies will take several decades. It may be the case that Neuralstem becomes the Medarex of stem cells. It could also be the case that other companies will develop superior approaches. It is extremely hard to predict biological evolution out one year let alone decades. What is clear is that Neuralstem at this point is clearly in the hunt and has to be perceived as the leader (along with Stem Cell) in neural stem cells.
Overview of Recent Stock Performance
Neuralstem first reported topline results for the phase 2 trial of NSI-566 stem cells for the treatment of ALS on March 12, 2015. At the opening, the stock traded up to about $4.15 in early trading from the close of $3.74 at the close on March 11. The stock was then was hit with an avalanche of short selling as 18 million shares traded on March 12 and March 13. (normal trading volume before then was running about 500,000 to 700,000 shares per day). The stock closed on March 12 at $2.37 and by the end of the month declined to $1.90.
The information in the press release was incomplete and did not give much detailed data. However, what was released left open some questions, the most important of which was whether the NSI-566 neural stem cells actually accelerated disease progression in some patients. More recently, in a September presentation in Sweden. The lead investigator Eva Feldman stated that this was not the case, but this concern has not yet been directly addressed by Neuralstem. Even though the phase 2 data when analyzed carefully was encouraging (Dr. Feldman and the other lead investigators agreed on this), it did not measure up to spectacular results seen in phase 1.
There is an agreement between companies and investigators that companies will not discuss in detail data from a trial until it has been presented at a major medical conference or published in a peer reviewed journal. As a consequence, Neuralstem has remained mute since March and the unanswered questions were used as ammunition for a short selling attack on the stock. As the stock was being drubbed, investors waited for more detailed information and focused on the presentation of lead investigator Eva Feldman at the American Neurological Meeting in September. Discouragingly, a press release summarizing her presentation again gave limited information and while providing a little more information, the presentation of the information was confusing. Once again the stock was hit with a barrage of short selling as 3.0 million shares traded (early in September the normal trading volume was running about 150,000 shares per day). The stock had closed at $1.58 on the day (September 28) before the press release. It closed at $1.38 on September 29 and at $1.22 on September 30.
I believe that Neuralstem was the target of a cartel of hedge funds who use naked shorting techniques to manipulate stocks of small biotechnology companies. One of their critical tactics is to make good news or any news appear to be bad by creating pressure on the stock. The poor stock behavior makes investors question the data. See my report Illegal Naked Short Selling Appears to Lie at the Heart of an Extensive Stock Manipulation Scheme for more detail.
The lead investigators on the study gave every appearance of being encouraged by the results. In the press release on March 12 that reported topline results for the phase 2 trial, three key opinion leaders involved in the trial described the results as positive and supportive of doing a next step phase 2b/3 trial. Investigators are now saying that this trial will start in the next few months (before yearend). Based on information I have gathered, this could be a 50 patient study with a control arm and will use ALSFRS-r scale as the primary efficacy endpoint similar to phase 2 and phase 1.
A sophisticated investor not involved with the stock would likely take the data presented thus far as being (very) positive. Discussion of the combined phase 1 and 2 data is the major purpose of this report. In a significant percentage of patients treated with the neural stem cells (I estimate 8 out of 14 or 15), the disease appears to have been stabilized and there are three patients from phase 1 whose ALSFRS-r has been stable for over four years.
ALS is a disease which inexorably progresses and only 5% of patients remain alive at 5 years and these surviving patients are sharply debilitated. These results can only be taken as a very strong signal of activity in ALS although the small number of patients treated so far prevents drawing firm conclusions. If it is shown in the upcoming phase 2b/3 trial that 20% to 40% of patients treated experience a stabilization or just a meaningful slowing of the disease progression, it could be viewed as a major medical advance. ALS physicians and patients are desperate for any therapy.
Thoughts on Commercial Potential
This therapy will not be applicable to all ALS patients. There is good evidence that it will not be effective in bulbar patients. Generally ALS begins in the lower (lumbar) region of the spine and progresses to the upper (cervical) region of the spine. In bulbar patients the opposite occurs. Bulbar patients are about 15% of the ALS population. The investigators also emphasize that the stem cells (after engraftment) do not grow new axons that control muscle function. Their mode of action seems to be that they release certain growth factors that nurture damaged neurons and protect neurons from further damage. It is likely that the NSI-566 stem cells will be primarily effective in newly diagnosed patients. It is also important to understand that the administration of NSI-566 stem cells involves a complex surgery in which the spinal cord is laid open and stem cells are then injected at intervals of about 4 millimeters on opposite sides of the spinal cord; this requires hospitalization. Because this is a transplant of allogeneic cells, immunosuppression (short term?) is currently required. Not all patients will agree to this complex surgery and immunosuppression.
The Center for Disease control estimates that there are 5,000 newly diagnosed cases of ALS each year in the US and the prevalence is 12,000. The question is what number of patients may benefit from this therapy and it will probably take many years to determine which patients may benefit most from the stem cells. My hypothesis is that it will be some percentage of the 5,000 newly diagnosed patients.
The next question to address is what might the price of the surgery be and what part of this cost would be reimbursement for stem cells. This will, of course, depend on the efficacy of the cells in a designated population. We all know that drugs for orphan diseases like ALS can be priced at $200,000 or more (depends on efficacy. We also know that the new checkpoint inhibitors, Opdivo and Keytruda, are priced at $140,000 per year and that the cost of allogeneic, hematopoietic stem cell transplant can be $200,000 to $300,000. For the sake of discussion, let’s say that the NSI-566 stem cells are priced at $150,000 for each surgery. This means that every 1,000 patients treated would result in $150 million of revenues for Neuralstem.
The next question is when might the NSI-566 stem cells be approved which, of course, depends on the clinical data. In the most positive case in which the results of the upcoming phase 2b/3 trial identify a population that could benefit from the drug, accelerated approval could be granted on the basis of just one trial. This is because ALS is as deadly and progressing as the most deadly cancer. There is no effective therapy and patients and caregivers are desperate for any hope in treating this deadly disease,
Dr. Eva Feldman and Her Insights
Her Credentials
Dr. Eva Feldman has been involved with the development of neural stem cells for over ten years beginning with the first studies in animals. She was also the lead investigator for the phase 1 and 2 clinical trials of Neuralstem’s NSI-566 neural stem cells in the treatment of ALS. She will also lead the upcoming phase 2b/3 trial. She has spoken extensively about her enthusiasm and hope for the potential use of neural stem cells in ALS.
Much of what is known about the studies of these stem cells comes from her public comments and medical conference presentations. She has impressive credentials as one of the leading ALS investigators in the world. Dr. Feldman is the recent President of the American Neurological Association. She is a Director of the A. Alfred Taubman Medical Research Institute and Director of Research of the ALS Clinic at the University of Michigan Health System. Dr. Feldman is an unpaid consultant to Neuralstem and owns no stock in the Company.
Dr. Feldman presented at a conference in Sweden on September 17, 2015 which I listened to carefully and I would others to as well. This gave an interesting overview of her efforts to find a treatment for ALS. She was at the forefront in hypothesizing that neural stem cells could be of value in treating neurological disease like ALS. Over the last ten years, she has overseen the development of neural stem from pre-clinical studies through the phase 1 and 2 trials in which she was the lead investigator. She went over this history at the conference and I think it adds great insight into interpreting clinical data that has been created.
Traditional Drug Development Efforts Have Resulted Only in Failure
She began by stating that the pharmaceutical industry in its attempts to develop drugs for ALS has taken a traditional approach. This is a mechanism based approach in which drug development is focused on interfering with and slowing the disease process. However, this requires an understanding of the disease pathway and where in this pathway intervention might slow, halt or reverse the disease process. The problem is that the degeneration of motor neurons in ALS and the resultant muscle atrophy and loss of muscle control is poorly understood. So far this has led to failure after failure in ALS drug development.
Earlier in her career she worked on an approach based on injection of the growth factor IGF-1. In addition to testing this potential drug in animal models, she was also an investigator in three double blind, placebo controlled trials. The last of these was a large, government funded trial involving 360 patients. The trial lasted for over two years and was one of the longest trials ever done in ALS. Unfortunately, the trial was a clear failure and this caused her to consider a new “out of the box” approach that was stem cell therapy which was based on a different precept. Basically, the idea was to put stem cells in the diseased area and see if they could cause the formation of synapses and protect still functional motor neurons from disease progression.
Why Neural Stem Cells Were Selected for Development
The starting strategy was to place stem cells in the diseased spinal cord areas in animal models of ALS to see if they could affect disease progression. At this point, the critical question was what type of stem cell should be used. Research began with embryonic stem cells which were obtained from eggs fertilized in vitro; these eggs otherwise would have been discarded. After about five days, researchers could take cells from an embryo and do a genetic analysis. It is at this early stage that totipotent cells develop that have the ability to become any type of cell in the body.
Cells are taken from the embryo and dispersed in a culture resulting in embryoid bodies which contain hundreds of thousands of cells. From this they can select embryonic cell lines that could then be expanded to billions of cells. Certain resultant cells were then injected into rat spinal cords to determine if they would integrate. What they found was remarkable and alarming. In about 5% of rats they saw the formation of significant tumors while in the other 95%, the cells would integrate beautifully and produce neural cells. However, these embryonic cells behaved in an uncontrolled way so that they not only differentiated into nerve cells but also formed cells found in teeth, hair and bone. Consequently, the embryonic stem cell approach was abandoned.
The next step was to look at induced pluripotent stem cells which came from an eight week old fetus (much later stage than embryonic stem cells) that had been donated to the National Institutes of health. Neural stem cells (neuroprogenitor cells) were taken from the spinal cord of that fetus. Unlike the embryonic stem cells that could become anything, these cells were set on a path in which they could only become some type of neural cell. They could become neurons or glial cells like astrocytes and oligodendrocytes. Dr. Feldman decided to go forward with a cell line which is now known as the NSI-566 neural stem cell line.
Studies in Pre-Clinical Models of NSI-566 Neural Stem Cells in Rodents
Research with NSI-566 stem cells began in small animal models (mice and rats); which had been genetically engineered to try to mimic ALS as it occurs in humans. These neural stem cells were injected into the spinal cord of a rat model of ALS. The results were exciting to investigators. They saw that the neural stem cells integrated with nerve cells in the spinal cord and made neurons which put out numerous axons. Dr. Feldman emphasizes that it is important to understand that these transplanted cells did not replace dead or dying motor neurons. In actuality, they surround the sick motor neuron, form synaptic contacts with them and nurse damaged motor neurons back to health or protect healthy neurons. She said that researchers have confirmed this mechanism of action in multiple ways.
In ALS, the spinal cord is highly inflamed and the neural stem cells appear to substantially reduce the degree of inflammation through a process that is not well understood. Importantly, she said that other research groups have confirmed her group’s findings. In a rat model, they injected live neural stem cells and dead neural stem cells as a control. There were dramatically more surviving motor neurons in the spinal cords of rats injected with the live neural stem cells and was also a significant increase in rat survival. There have been hundreds of experiments in different rodent models of ALS that confirm this. After five years of work, she was also confident that there was no tumor formation as with embryonic cells and that animals injected with neural stem cells lived longer. She re-emphasized that investigators don’t fully understand the mechanism of action,
Moving on to Larger Animals-the Mini-Pig
The success in rodent models allowed them to go forward in larger animals. They chose to use the mini-pig which has a spine quite similar to that of a human. They performed over 100 surgeries which showed that it was quite safe to inject cells into both the lumbar and cervical areas of the spine at intervals as close as 2 mm. Importantly, they developed a technique that allowed them to follow the course of the neural stem cells over time. The cells were labeled with ferritin labeling which allowed them to be distinguished and visualized with MRI. Sequential MRIs could determine where the stem cells wind up in the spinal cord.
The starting hypothesis was that the neural stem cells would migrate up and down the spinal cord and give enhanced protection. However serial MRIs of the labeled cells showed that they didn’t migrate. There was initial disappointment in this finding, but others suggested this might be a positive because migratory stem cells might have more of a tumorigenic potential. Another very significant result was that the surgeries were safe and that pigs did quite well following injections in both the cervical and lumbar region of the spine. Remember that these cells require a complex surgery for injection.
Studies in Man Could Begin
After this extensive work in animals that spanned five years, the FDA approved an IND to begin human studies. In 2009, the first injection of neural stem cells was made into the spinal cord of an ALS patient. This marked the beginning of the start of human trials in neurodegenerative diseases using cellular therapies. These studies will not be confined to just ALS. They are doing comparable studies to those done in ALS with mouse models of Alzheimer’s disease. The next step will be large animals; the large animal model for Alzheimer’s in Macaque monkeys instead of mini-pigs. Like ALS, the underlying causes of most neurodegenerative diseases is not well understood. In ALS the environment in which the cells are injected is inflammatory and the hope is that this area can be settled down. Dr. Feldman described this as going into a really bad neighborhood and making it a really good one.
Design of the Phase 1 Trial in ALS
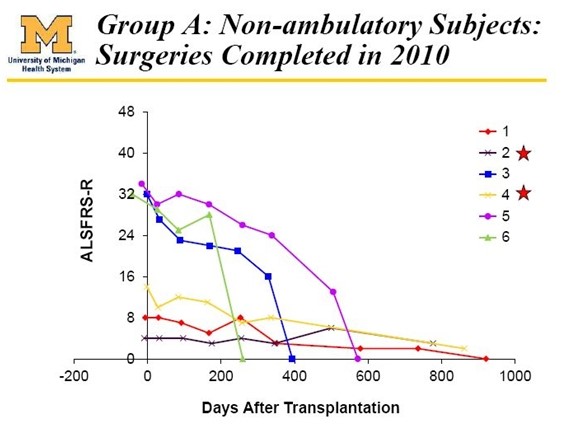
The phase 1 trial that began in 2009 involved 12 ALS patients but there were 15 surgeries because 3 patients underwent a second surgery. This was a risk escalation trial whose primary goal was to determine if the neural stem cells could be safely transplanted into human spinal cords. The first 6 patients were extremely advanced, paraplegic ALS patients who almost certainly had nothing to gain, but also nothing to lose if the surgeries went bad.
These first 6 patients received injections in the lumbar (lower) portion of the spine which were intended to establish that neural stem cells could be safely injected into the spinal cord of humans. After establishing safety, the next 6 patients were earlier stage ALS patients who were ambulatory and much more functional. These patients could walk into the hospital to get their transplants. In this early on course of the disease there might be only a little hand and leg weakness and nothing else.
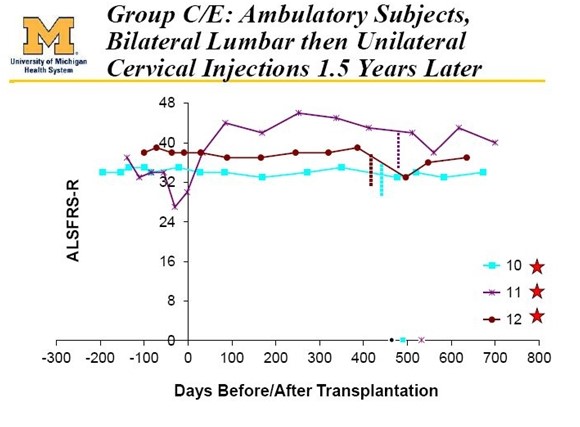
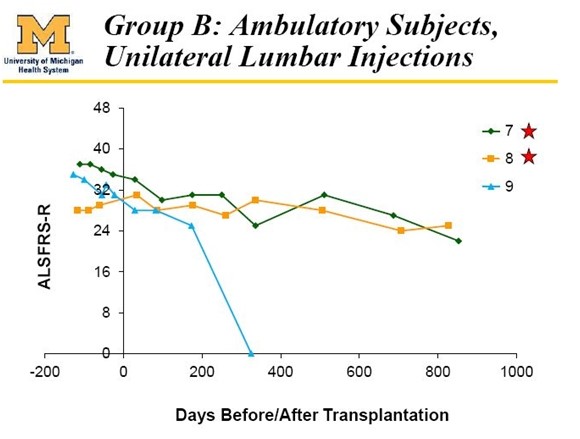
These last 6 patients had a lot to lose if the therapy went bad. Because of this the FDA mandated that they proceed very slowly in these 6 ambulatory patients. In the first 3 ambulatory patients, they began with unilateral (on one side of the spinal cord) injections in the lumbar section of the spinal cord as was done with the 6 paraplegic patients. In the next 3 ambulatory patients, they went on to bilateral (both sides of the spine) injections in the lumbar region. Finally, these latter 3 patients were brought back one year after their initial surgery and were given injections in the cervical region.
The FDA imposed conditions which resulted in the phase 1 trial taking over three years to complete. The FDA would only allow investigators to do one patient and then wait one month before doing the next. They did cohorts of three patients and they also had to wait one to two months between cohorts (each cohort had 3 ALS patients) as the FDA carefully examined the data. Dr. Feldman was frustrated by this slow pace but in retrospect recognizes that it was the right way to proceed.
The phase 1 trial was conducted in five different cohorts (A, B, C, D and E) of three patients as follows:
- Cohort A: 3 non-ambulatory patients received five unilateral injections of 0.5 million cells per injection in the lumbar region.
- Cohort B: 3 non-ambulatory patients received ten bilateral injections (five on each side of the spine) of 1 million cells per injection in the lumbar region.
- Cohort C: 3 ambulatory patients received five bilateral injections of 0.5 million cells per injection in the lumbar region.
- Cohort D: 3 patients received ten bilateral injections of 1 million cells per injection in the lumbar region. These patients were brought back one year later and given five unilateral injections of 1 million cells in the cervical region.
The Transplant Procedure Requires Complex Surgery
The injections of these stem cells requires exposing the spinal cord so that the cells are injected directly into the cord. Because the spinal cord is moving with the body as the surgery is performed, a surgical technique and apparatus had to be developed that could precisely deliver the cells. The solution was to do a laminectomy in order to access the spinal cord. To stabilize the device they drilled surgical posts into the lamina above and below the area of the surgery to which was attached a surgical device which then moves with the spinal cord of the patient. There is then a floating cannula that can move along a track up or down the spine to allow for serial injections into the patients. The spinal cord is moving throughout the procedure and this is why it is so important to be tethered to the patient. This is everyday stuff for the neurosurgeon, but quite new for the neurologist.
This is a major surgical procedure; it is not just a doctor using a needle to inject cells into the back. During the procedure, the patients are given extraordinary monitoring. Interestingly. Dr. Feldman said that government (presumably FDA) employees were sometimes present at surgeries. The stem cells were received from Neuralstem the morning of the surgery having been prepared the night before. One of the critical decisions in the surgery in addition to determining where to inject (lumbar or cervical, unilateral or bilateral) was the spacing between injections. In the mini-pig model the injections could be safely done at 2 mm. intervals but in humans the spacing was 4 mm. After exposing the spinal cord, they then confirm the positions for injections using a simple ruler.
Measuring Safety and Efficacy Results from Phase 1
The patients were scrupulously followed initially and then on an every two week basis. All of the first six non-ambulatory patients who were treated have died. However, others have been followed for up to 4 1/1 years from their initial surgery. Almost all Phase 1 trials, regardless of disease state, are primarily safety trials. This trial incorporated multiple measures of safety. The investigators concluded that the procedure was safe but there were adverse events which were mainly characterized as minor.
There was also a secondary goal of determining efficacy. They used the ALSFRS-r scale which is a qualitative scale administered by the patient’s neurologist. This is a 40 point scale measured in 8 domains that assesses respiratory, motor and bulbar function. It is the FDA recognized primary endpoint for ALS studies. They also used various measures of respiratory capacity, quantitative strength testing, spasticity, electrical impedance myography, and quality of life. The trial was powered for safety, not efficacy.
The first six patients have all died which allowed investigators to perform autopsies to examine the effect of the injections on the spinal cord and the fate of the injected stem cells. Dr. Feldman said that the spinal cord looked beautiful and she doubted that anyone could know by looking that there has been stem cell injections. They sliced the cord and performed immunohistochemistry, PCR and DNA analysis of the spinal cord. In this advanced disease state, there was no indication that existing motor neurons had been preserved. They were able through DNA analysis to determine that some of the injected stem cells had survived. They could find no indication that the surgery accelerated the progression of the disease.
The injected cells are allogeneic cells which raises the concern that they could cause an immune reaction. This is based on experience with solid organ transplants from adult donors However, this is not a full organ transplant and there is a general belief that fetal stem cells have much less potential to cause an immune response than cells from a transplanted adult organ. Nevertheless, all patients were immuno-suppressed with the drugs mycophenolate mofetil or tacrolimus. Side effects from these drugs and the immunosuppression that they cause was the major reason for adverse events in phase 1.
Dr. Feldman concluded that in some patients, these side effects were such that she felt that whatever the potential benefits were, she could not justify keeping the patients on the drugs. She took all of the first six patients treated off immunosuppression. One was off for 8 months and another for 9. Despite this the injected stem cells remained in the spinal cord and were not rejected; this was determined by autopsy. She feels that they may not have to do long term immunosuppression and perhaps treatment could be limited to three months.
A Detailed Look at the Clinical Data from Phase 1
Neuralstem has now conducted a phase 1 trial and a phase 2 trial. The phase 1 trial was very carefully conducted with the FDA watching every patient. This was the first trial of Neuralstem’s NSI-566 neural stem cells in ALS. The FDA was hyper-concerned because previous animal studies with embryonic stem cells (not neural stem cells) showed that they differentiated wildly and unpredictably into several different types of cells and in perhaps 5% of rodents treated could cause cancer. Understandably, the FDA and investigators in the phase 1 trial were focused on safety above all else.
The first six patients ([patients 1, 2, 3, 4, 5, 6) treated in the phase 1 trial were very advanced paraplegic patients in advanced stages of ALS. There was virtually no hope or expectation that the stem cells would help them but they volunteered to undergo the surgery in the hope that they might help future ALS patients. The sole intent was to show that the cells could be safely implanted and this was the indeed the result. There was no indication of efficacy in these patients as can be seen below:
The study then went on to dose 6 more patients who were more healthy and in a much earlier stage of the disease. There were startling signals of efficacy in these patients even though they were injected with a number of cells that was thought to be sub-optimal based on animal studies.
These six patients were divided into cohort C (patients 7, 8, 9) and cohort D (patients 10, 11 and 12). Each of the patients in Cohort D achieved results that can quite honestly be called amazing. The FDA accepted measure of efficacy in ALSFRS-r is a 48 point scale using six quadrants. A perfectly healthy patient scores 48 on the scale while most newly diagnosed ALS patients score around 40. Considerable historical experience in several hundreds of patients shows that ALSFRS-r scores decline fairly steadily at perhaps xx points per month.
Death usually occurs when the ALSFRS-r score reaches 15 to 20 which can take two to three years from diagnosis. Over this period of time, patients suffer from continuing degradation of bodily functions, first losing control of their legs and arms and ultimately die from respiratory failure as muscles controlling breathing fail. Only about 5% of ALS patients survive for 5 years although there are some atypical patients such as the famous British physicist Steven Hawking who decline to a low functional status and then the disease seems to burn itself out. In all patients, quality of life drops sharply.
Results in patients 10, 11 and 12 were extraordinarily positive and investors were made aware of the results because patient 12 was an active blogger named Ted Harrada who provided investors a detailed report on his success in the trial. All three of these patients have survived 4 1/2 years after treatment began (remember that about 5% of ALS patients survive 5 years from diagnosis.) However, their ALS score have been roughly flat over this period which is exceptional. The latest chart showing this is as below:
The Results in Cohort C (patients 7, 8, 9) were also very positive. Patient 9 died of a heart attack which was determined by the investigator to be not attributable to the stem cell treatment. The last reported results show that patients 7 and 8 also experienced meaningful benefit as is shown below:
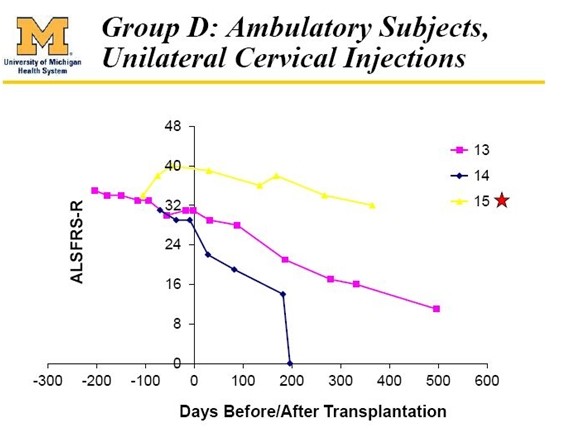
Also treated in the phase 1 trial were three bulbar patients (13, 14 and 15) who differed from all others in the trial as they presented with bulbar symptoms which are due to deterioration in nerves in the cervical regions of the spine. These nerves control functions such as breathing, eating and speech. ALS symptoms usually start with deterioration of nerves in the lumbar region that then progress over time to the cervical region. Patients with bulbar symptoms have a different course of disease progression that is more rapid for reasons that are not well understood. This is shown dramatically in the following table. These bulbar patients are not included in the effe analysis of phase 1.
With the important and necessary caveat that phase 1 dealt with a very small number of patients, the results suggested that all five of the patients who might have some hope of benefitting did so and the results are extraordinary. I exclude the six paraplegic patients (1, 2, 3, 4, 5 and 6), patient 9 who died of a heart attack and patients 13, 14 and 15 who were bulbar patients.
The Trial Design for Phase 2
According to Dr. Feldman, the initiation of the phase 2 trial was a much easier process than phase 1 as it required only two applications for trial approval versus six for phase 1. The FDA had more confidence that clinical experience gained in phase 1 indicated that both lumbar and cervical injections delivered bilaterally could be safely given. There were two major differences in phase 2 from phase 1: (1) an emphasis on cervical injections and (2) giving greater numbers of cells per injection.
The motor neurons arising in the cervical region of the spine control respiration while those in the lumbar region are more involved with legs and arms. Because most patients with ALS die because of respiratory failure or complications, the thought was that cervical injections were more likely to result in improvement in mortality and provide higher quality of life. Right from the start, the phase 2 trial began with bilateral cervical injections. Another major difference was that instead of 100,000 cells per injection, they initially used 200,000 cells and then 300,000.
The phase 2 patients like the final 6 phase 1 patients treated in phase 1 were all quite functional. Each had good leg and arm use and had to have an FVC (forced vital capacity) of at least 70%. These were very functional patients. The trial enrolled 15 patients in cohorts of 3 and every patient had roughly the same functional status. The dosage per cohort was as follows:
- Cohort A (patients 310, 320, 303): There were ten bilateral injections in the cervical region for a total of 2,000,000 cells.
- Cohort B (patients 304, 305, 306): There were 20 bilateral injections in the cervical region for a total of 4,000,000 cells
Dr. Feldman said that patients in cohorts A and B all did fine. Based on this, she concluded that the plasticity of the spinal cord in being able to handle this number of injections was remarkable. However, she couldn’t see increasing the number of injections so they decided to the number of stem cells per injection from 200,000 to 300,000.
- Cohort C (patients 307, 308, 309): There were 20 bilateral injections of 300,000 cells each for a total of 6,000,000 cells.
- Cohort D (patients 310, 311, 312): There were 20 bilateral injections of 400,000 cells each for a total of 8,000,000 cells
- Cohort D (patients 313, 314, 315): In these final three patients they went back and did lumbar injections. They did 20 lumbar injections of 200,000 cells for a total of 8,000,000 cells and one month later did 20 cervical injections of 200,000 cells for a total of 8,000,000 cells. Altogether these patients received 16,000,000 cells.
There was also a difference in surgical approach in which the anterior posts were actually fixated to the skull because they couldn’t fixate to the lamina. The posterior posts remained fixated to the lamina. The cervical injections are technically more challenging. The area is a little smaller and there is a lot more pulsation of the spinal cord. They also had to be cautious of nerve roots emanating from the area and this area is also much more vascular than the lumbar section. Nevertheless, they were able to clear a robust area in order to do injections. She said it is a challenge to make 20 cervical injections given the vascular and other constraints, but it can be done.
Dr. Feldman’s Comments on Phase 2:
Dr. Feldman states that the surgeries have gone beautifully and that you physicians probably can safely make 20 bilateral injections in the cervical section of the spinal cord with 300,000 cells per injection. They are doing the injections 4 millimeters apart; in the mini-pig model, they were able to do them 2 millimeters apart. As with phase 1, determination of safety was the primary objective of the trial.
Unlike phase 1 which took over three years to complete, the FDA did not require having to wait a month or more between surgeries. They were able to do a surgery per week in each cohort. Then they would wait about two to four weeks to begin the next cohort.
They expanded from one clinical trial site in phase 1 to three sites in phase 2. At one of the new sites, following surgery one patient experienced increased weakness of the legs and arms to an extent that had not been seen in other patients. This surgery was in cohort C in which the patient got 20 bilateral injections of 300,000 cells each. The patient was in a rehab facility for approximately three months. Dr. Feldman said that he has since recovered to the point that one would expect at this stage of ALS. It seems probable that the issue was surgical technique rather than the stem cells.
She said that immunosuppression is the bane of her existence. She believes that they may have enough data to show that they don’t have to immunosuppress beyond 3 months in future trials. This immunosuppression appears to cause side effects which almost certainly affect the ALSFRS-r score which relies extensively on measures of quality of life. Troublesome side effects from immunosuppression would likely have a negative effect.
In looking at all of the data from the phase 2 trial she says that she is very excited about it. She and Neuralstem are discussing with the FDA a phase 2b/3 trial that will likely begin before yearend 2015. The objective of that trial will likely be to show a slowing of progression of the disease.
Analysis of Phase 2 Data
The announcing of the phase 2 results in March 2015 have been accompanied by nearly a 75% decline in the stock price. This is unexpected as I think that no sophisticated investor looking at the company for the first time would think that the results were discouraging. As I will shortly touch on the results were not as striking as in phase 1, but were still encouraging. I think that part of the reason for the decline is that even though when viewed objectively, the phase 2 results were positive and encouraging, they did not come close to measuring up to the spectacular results of the 5 patients in phase 1.
In addition, Neuralstem has done a very poor job in presenting these results. The accepted practice for a company is to only release topline results and then present detailed results when an investigator publishes in a peer reviewed journal or presents at a major conference. In March 2014, when the topline results were released it was clear that they were not as striking as those of phase 1 and it could be interpreted that the NSI-566 stem cells may have actually accelerated the course of the disease. Eva Feldman says this is not the case. Neuralstem however remained mute on a detailed interpretation of the results offering no explanation as to why some patients declined so sharply.
Along with most investors, I thought that Eva Feldman’s presentation at ANA in September would go into great detail so that we could make a clear interpretation of results. However, at this point the only information on her presentation was a press release that contained little new information and left open the question as to whether the NSI-566 stem cells were actually hurting some patients. I am now awaiting the publication of results in a peer reviewed journal which I hope will give a better insight into the study. I also think that following this, Eva Feldman will likely make some presentations that will provide meaningful additional insight on the NSI-566 stem cells.
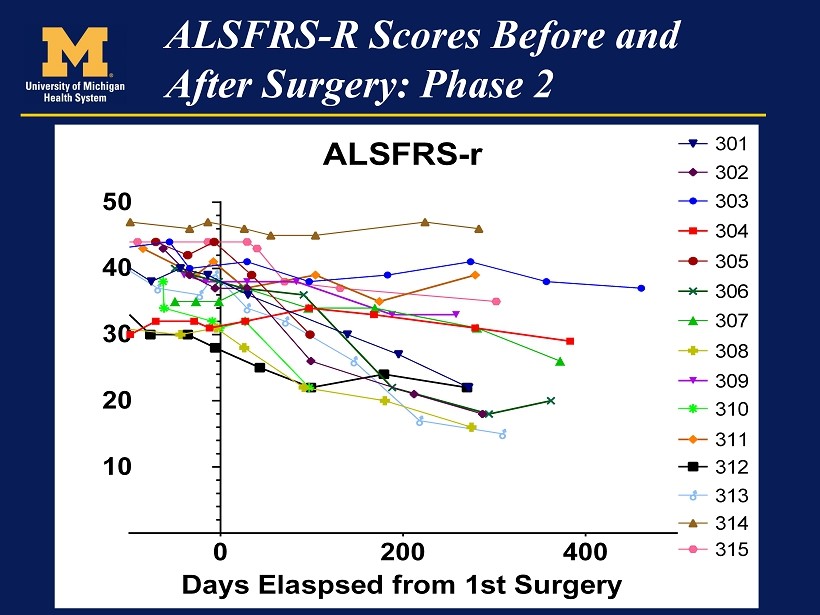
At this point the data from phase 2 has been presented in a manner that is very had to understand and there has been no comment from the Company. I have made several calls to the Company that have gone unreturned and aside from the somewhat terse press release, they have not commented on results. Here is what we have to work with at this time. Following the presentation by Dr. Feldman, Neuralstem released an 8-K which contained slides relating to her presentation. The key slide relating to the data was as follows:
I found this visual representation is difficult to interpret. The lines overlay each other especially in the first 100 days of therapy and it is difficult to put the outcomes of these patients in perspective. I looked at the graph showing ALSFRS-r scores in phase 2 and tried to estimate ALSFRS-r scores at various points in time by “eyeballing” it. This was very difficult and prone to error, but this is all I have right now. I estimated (by eyeball and ruler) ALSFRS-r at three time points:
- The first measurement before baseline
- Baseline (day 0), the time of surgery
- At the end of the measurement period which is not fixed
I also estimated the time following synergy for each patient. By taking the difference in ALSFRS-r at the time following surgery from baseline and dividing this number by the number of days following surgery allowed me to estimate the change in ALSFRS-r per day. I then compared this to historical data showing rate of decline of control patients in previous ALS trials.
In its press release, Neuralstem stated that the expected rate of decline in ALSFRS-r was -1.2 points per month (-0.040 per day). In Cytokinetics BENEFIT-ALS trial of tirasemtiv, the rate of decline of ALSFRS-r over a three month period was -0.8 points per month (-0.027 points per day). By using these two estimates I was able to calculate what the expected range of ALSFRS-r score would be for each patient at the end of each period. The results are shown in the following table.
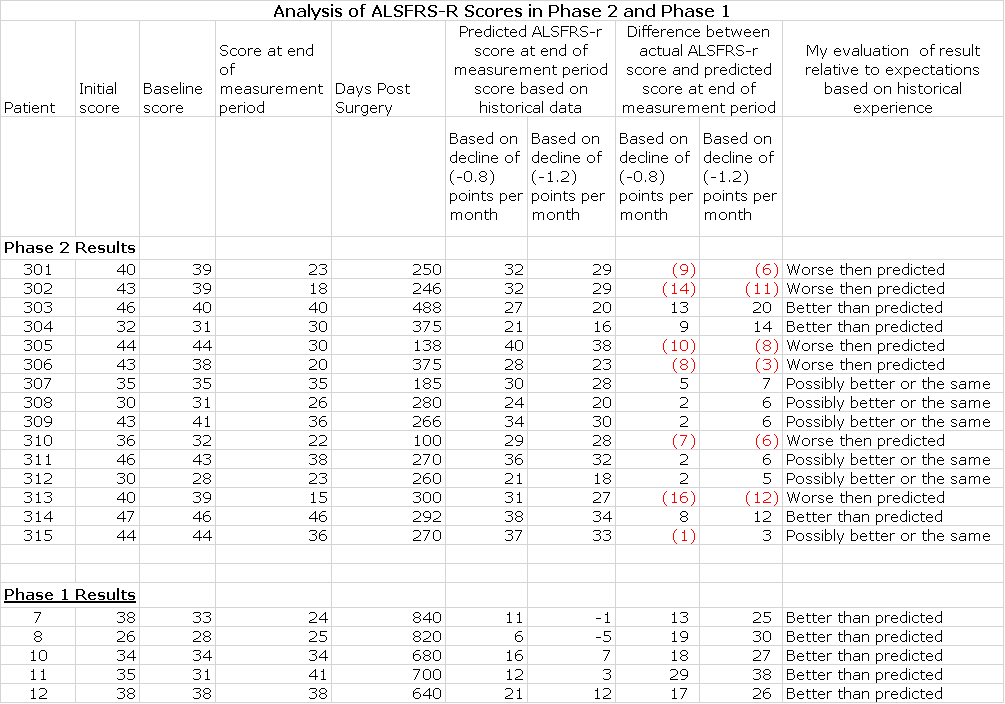
I would urge you take look at this table with some skepticism. It suggests that 3 patients in phase 2seem to be showing ALSFRS-r scores substantially better than historical controls and hinting at stabilization comparable to the 5 patients in phase 2. These are patients 303 from Cohort A, 304 from Cohort B and 314 from Cohort E. Six patients appear to have done worse than expected and 7 seem to be showing ALSFRS-r scores somewhat better or in line with historical controls. Patient 313 was identified by EVA Feldman as a patient who suffered a serious adverse event and the decline in ALSFRS-r may not be indicative of the effect of the stem cells.
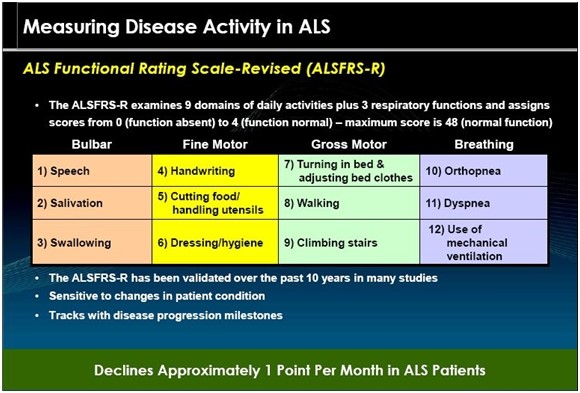
The ALSFRS-r Scale
The ALS Functional Rating Scale is the most important measure of the degree of impaired functionality caused by ALS and can also track the progression of the disease. It combines nine criteria involved with daily activities and three involved with respiratory function. It is based upon a subjective measurement by the physician or patient for each function. A score of 0 indicates that there is an absence of the function and a score of 4 means that there is normal functionality. The 12 elements of the scale are shown below:
A normal person would score 48 and a person with ALS would score about 36 a year or so after diagnosis. There is a persistent decline in the ALSFRS-r score of about 1 point per month. Key opinion leaders state that while there can sometimes be a period of stability of a month or two, there is an inexorable decline and sustained improvements just don’t occur. Death usually occurs when ALSFRS-r reaches 16 to 18. Death is almost always the result of respiratory failure or complications such as infections that can occur when breathing must be assisted by mechanical ventilation.
Tagged as CUR, Inc., Neuralstem, Inc., NSI neural stem cells used to treat ALS + Categorized as Company Reports





Larry,
Thanks for your insightful update on the ALS program. Do you have any updates on the calif spine, China stroke, or depression trials? Seems like if there was any good n Artews it would leak out the way it did in the ALS phase 1 trial. Art
I Happen to agree with your theory about the ALFRS scores not really reflecting the majority of the patients who only received the cervical surgeries. The trial was focused on treating the breathing functions of these patients. If 6 parts of the ALSFRS functions are made up of muscle functions that are associated with the lumbar part of the spine, how can these ALSFRS graphs accurately predict the breathing functions that are associated with the cervical part of the spine?
Larry – what are your thoughts on management’s strategy of complete silence? No trial updates, no PRs, no returned phone calls, etc, etc. We are now dipping to the $1 mark on what appears to be a steady descent to zero. Incomprehensible, negligent & possibly criminal. Why would they continue to do this?
Neuralstem has respected the implied agreement between companies and investigators that it will not discuss data in detail until it has been presented at a conference and published in a peer reviewed journal. This silence has allowed the shorts to cream the stock. The data should be published soon and we could/should see details of the upcoming phase 2b/ 3 trial before yearend. I also expect Eva Feldman to speak publicly and with enthusiasm about results in the phase 2 trial.
A fellow investor reports that IR told him this week that they still expect phase 2 189 to begin in 2015, as well as phase 2 in stroke trial in china, noting that 3 additional patients had been dosed with a slightly different approach in China recently.
On 11/9 the Co. reported
“NSI-566 human neural stem cell therapy, under development for the treatment of ALS: Eva Feldman, MD, PhD, Director of the A. Alfred Taubman Medical Research Institute and Director of Research of the ALS Clinic at the University of Michigan Health, presented nine-month Phase II and combined Phase I and Phase II data at the American Neurological Association (ANA) Annual Meeting in September. The data showed that the intra-spinal transplantation of the human neural stem cells (NSI-566) was safe and well-tolerated. There appeared to be no acceleration in disease progression due to the therapeutic intervention with NSI-566. The Company is currently in discussions with the FDA for a larger, controlled, registration directed clinical trial. – See more at: http://investor.neuralstem.com/2015-11-09-Neuralstem-Reports-Third-Quarter-2015-Financial-Results#sthash.1kJOaiAM.dpuf”
This seems to lay to rest the uncertainty which caused the sp decline.
Isn’t it strange that the market has not picked up on this positive news?