Cytokinetics: I Think That the Company May Decide to Do A Phase 3 Trial with Tirasemtiv (CYTK, $4.70, Buy, For Subscribers Only)
Overview on Clinical Status of Tirasemtiv
I believe that Cytokinetics (CYTK) will decide to move tirasemtiv into phase 3 registration trials and that this decision will likely be announced later this year or early next. After listening to the conference call on April 30 that discussed the disappointing results in BENEFIT-ALS, it was my feeling then that Cytokinetics still strongly believed in the drug. After listening carefully to Robert Blum’s presentation at the Jefferies conference on June 4, I became even more convinced. He said “Tirasemtiv could be the next major advance in ALS” and he went on to say that the Company will be talking with key investigators about the next logical step forward for tirasemtiv.
A decision to move forward will be met with skepticism by many investors. In the phase 2 BENEFIT-ALS trial, tirasemtiv failed to reach the primary endpoint of showing a lesser rate of decline in the ALSFRS-r scale than the control group. In fact, patients on tirasemtiv showed a slightly greater decline in ALSFRS-r than control patients although this did not reach statistical significance. Over a three month period the average decline in ALSFRS-r for tirasemtiv patients was 2.98 versus 2.40 for placebo. This caused most investors to write off tirasemtiv and place most of the investment value of the Company on omecamtiv, its heart failure drug that could begin phase 3 trials in 2H, 2015.
Failure in a clinical trial does not always mean that the drug is ineffective. Trial design can sometimes be the cause of a trial failure that can be corrected and lead to success in subsequent trials. The recent experience with Acadia Pharmaceutical’s (ACAD) pimavanserin is an example of this. Two phase 3 attempts failed which caused the stock after the second failure to drop to $0.67 in November 2011. Then a well-designed phase 3 trial based on knowledge gained from the two previous trials resulted in a successful outcome and the stock is currently trading at $23.05. I would caution that the Acadia example is far from the norm. However, it does show that it is possible to use information gained in a failed trial to design a new trial that goes on to be successful.
There is and should be appropriate investor skepticism when a trial fails and a company opts to continue drug development. Failure in the trial means that the hypotheses that management used to conduct it were wrong or failed to take key factors properly into account. History has shown that small biotechnology companies are reluctant to give up on a drug for both emotional reasons. As a result, they will often perpetuate development of a drug that is doomed to fail. It is always a hard judgment for investors to make as to whether management has a reasonable basis for continuing development of a drug following disappointing trial results. This report examines, what I believe, are the principal factors in management’s thinking.
Should Cytokinetics Advance Tirasemtiv to Phase 3
The primary justification for going into phase 3 is that two prospectively defined secondary endpoints showed statistically significant improvements in the trial. Respiratory failure is the primary cause of death in ALS patients and a breathing measurement called slow vital capacity (SVC) which measures the amount of air that can be slowly exhaled after a deep breath is the most widely used and relied upon measure of breathing function. Survival is closely correlated with SVC and it is used for decision making on whether to put a patient on ventilatory assistance, to surgically intervene or to decide to put a patient in hospice.
In this phase 3 study, the rate of decline in SVC was measured similarly to the rate of decline in ALSFRS-r. Here the results were strikingly positive. The rate of decline in SVC for tirasemtiv patients was only one-third that of control patients and the p value was a very strong p=0.0006. This strongly suggests that by slowing the rate of decline in breathing dysfunction that tirasemtiv could have an effect on median overall survival.
The second encouraging results for a proscribed secondary endpoint were based on Mega muscle strength which is a composite measure of the decline in strength in five muscle groups on each side of the body. Deterioration in muscle strength is the unfortunate hall mark signature of ALS. On this measure, tirasemtiv slowed the rate of decline to one-third that of the control group and the p value was a statistically significant 0.0158.
On a negative note, results were not statistically significant for the secondary endpoints of SNIP and MVV which were two other measures of respiratory function. Key opinion leaders believe that these are less reliable indicators of breathing function. Another secondary measure of muscle strength is grip strength which showed a numerical improvement for tirasemtiv but was not statistically significant. So the secondary endpoints of the trial showed mixed results.
In summary, tirasemtiv missed on the primary endpoint of ALSFRS-r and three secondary endpoints. However, it can be argued that the secondary endpoint of SVC is the most important variable to look at because it is correlated with survival. Also, the effect on Mega-muscle strength is very encouraging in suggesting a slowing in deterioration of muscle strength. Cytokinetics believes that tirasemtiv is the only drug that has ever demonstrated the ability to slow the decline in respiratory function and muscle strength in a large (over 200 patients) clinical trial.
The side effect profile of tirasemtiv may have played an important, negative role in the outcome of the trial as the drug is not well tolerated in ALS patients. There were 303 patients randomized to tirasemtiv in the trial and 73 dropped out due to side effects as compared to 302 control patients of whom 12 dropped out because of side effects. In the tirasemtiv group 96.7% of patients reported adverse events versus 87.5% in the control group. The side effects that occurred most frequently for tirasemtiv were dizziness (50.8% of patients), fatigue (33.2%) and nausea (21.9%). Those side effects which occurred more frequently with tirasemtiv were dizziness (31.2% difference in incidence from placebo), fatigue (19.0%), nausea (14.1%), muscle spasm (9.5%), confusional state (9.9%), decreased appetite (6.9%), headache (6.8%), and insomnia (6.2%).
I will discuss the ALSFRS-r scale in more detail later but it is essentially made up of 12 different factors that determine quality of life for an ALS patient. Each of these is evaluated on a scale of 1 to 4 by the patient or the physician to determine the final score. It is highly probable that the side effect profile of tirasemtiv resulted in patients feeling worse and negatively affecting the resultant ALSFRS-r score. There was also a negative effect of tirasemtiv in causing weight loss and in prior clinical trials of other drugs this has correlated with a poorer outcome. The study showed that weight loss at each week of the three month trial was twice as great on tirasemtiv as control. Another indicator of the impact of side effects on the trial is that it was difficult getting patients to the highest dose. In the clinical trials only 50% of patients on tirasemtiv could tolerate the highest dose of 250 mg BID.
I think that CYTK and key opinion leaders believe that tirasemtiv had a clearly positive biological and clinical effect on ALS. This is the first drug that has shown a positive effect in slowing the rate of decline in respiratory failure and loss of muscle strength. The question is can the side effect profile be handled more effectively and keep more patients on the drug. The answer to this question will determine if a new phase 3 trial should be started.
What Might a Potential Phase 3 Trial Look Like?
If CYTK is going to go forward into phase 3, it seems to me that it might try to persuade the FDA that SVC would be a valid primary endpoint. This would be a major shift for the FDA because the ALSFRS-r scale has been validated in clinical use and used as the primary endpoint in ALS clinical trials for a long period of time. The FDA might want something more than just SVC such as median overall survival. Cytokinetics will probably want to get a Special Protocol Assessment with the FDA that it would accept SVC or some other measure other than ALSFRS-r as a primary endpoint.
Cytokinetics will also try to improve the side effect profile so that patients stay on the drug longer. In the BENEFIT-ALS trial, most of the dropouts on tirasemtiv occurred in the first four weeks. If better control of side effects could be achieved, it might improve trial outcomes. This also could possibly be done just by encouraging patients to stick it out for the first four weeks or to use drugs to control the side effects and weight loss. Neither of these measures was used in BENEFIT-ALS. This might also allow patients receiving tirasemtiv to be treated at the highest dose of 250 mg BID. In BENEFIT ALS only 50% of patients reached this highest dose.
There are a couple of other issues to think about that would affect planning of a phase 3 trial. Would it be wise to run a small open label phase 2 trial of tirasemtiv to see if either behavioral or drug intervention could reduce the dropout rate and increase the tolerated dose level of the drug? Maybe. Should CYTK conduct a phase 3 trial if the FDA insists on using ALSFRS-r as the primary endpoint? Unless, CYTK can find a way to dramatically reduce side effects, I would be somewhat skeptical on this course of action.
Investment Thesis
I think that it will take CYTK some time to reach a decision on how it wants to go forward and then to discuss and agree on the trial protocol with the FDA. They will probably want to get a Special Protocol Assessment. I don’t have a good estimate on how long this might take, but I would not be surprised if takes until late 2014 or early 2015.
I think that the initial reaction of the market to a new phase 3 trial for tirasemtiv in ALS would be cool or lukewarm. However, there is very little in the current stock price for the potential that tirasemtiv could be successful in a new phase 3 trial. I think that CYTK has a reasonable case for going forward and as CYTK more forcefully states its reasons, investors will listen and then begin to build some expectation into the stock price that a new phase 3 trial of tirasemtiv just might work.
Tirasemtiv is a flawed drug because of its side effect profile, but it is the only drug that has shown evidence of a positive therapeutic impact on slowing the rate of decline in breathing and skeletal muscle function. There is a desperate need for a new drug even if it is flawed to give ALS patients and their family friends and caregivers some hope. Perhaps this will cause the FDA to be flexible in its thinking on a phase 3 trial for tirasemtiv. I think that if the FDA accepted SVC as the primary endpoint of the phase 3 and agreed to a Special Protocol Agreement that it would provide a major boost to the stock price.
Tirasemtiv could re-emerge as the second leg of the CYTK investment platform. The first leg is omecamtiv mecarbil that is being studied in congestive heart failure. CYTK and its partner Amgen are currently enrolling 450 patients in the expansion phase in a phase 2 study called COSMIC-HF. This trial has no primary endpoint but is designed to give a better understanding of the oral formulation of omecamtiv mecarbil-is safety, tolerability and PK/PD effects. Topline data will be available in early 2015 and this will be followed by an end of phase 2 meeting with the FDA. Around this time, Amgen will make a decision on whether to move into phase 3; this would be a major positive
The potential third leg of the investment stool is CK-107. It affects the contractility of skeletal muscles similar to tirasemtiv. It is partnered with Astellas and is being studied in non-neurological disease in which increased muscle strength is desired. Multiple phase 2 studies are planned to begin in 2014. It is possible that this could be a backup drug for tirasemtiv in ALS although Cytokinetics, which has total ownership of tirasemtiv, would only allow this move if it gave up on tirasemtiv. Phase 1 studies suggest good tolerability for CK-107.
In looking at the stock price potential for Cytokinetics, there is no truly monumental event such as a binary trial outcome in 2014 or 2015 that could dramatically affect the stock as the BENEFIT ALS trial did in April 2014. I see two significant events through the end of 2015 that are likely to be meaningful catalysts for the stock. The first is likely to be an announcement on the status of a new phase 3 trial for tirasemtiv. If the FDA agrees to the primary outcome measure of slow vital capacity, this would be a major positive. However, the FDA may balk at using this end point and if the FDA insists on using ALSFRS-r as the primary endpoint. CYTK might abandon the drug and switch its ALS studies to CK-107.
A decision by Amgen to progress omecamtiv mecarbil into phase 3 would also be a major positive as it would trigger the first of what could be over $300 million of pre-commercial milestone payments to CYTK as well as validating the promise of the drug. I think that the potential for omecamtiv justifies owning the stock at these levels.
The timetable for the commercial introduction of omecamtiv mecarbil and tirasemtiv assuming success in phase 3 trials that are not yet underway suggests that they could be approved in 2018 or 2019. Hence, the performance of the stock will be based initially on the decisions to move forward into phase 3 on tirasemtiv and omecamtiv. Assuming that this decision to move forward is made and that the market (perhaps after some initial hesitancy) becomes positive or excited about both drugs, I think that having two drugs with such significant market potential could justify a $500 million market capitalization by late 2015 which compares to the current $228 million. This suggests a one year price target of $9 to $10 per share.
Financial Considerations
Cytokinetics has 36.1 million share of stock outstanding. There are 1.1 million warrants exercisable at $9.90 and 6.6 million at $5.28. There are also 4.9 million options and similar instruments outstanding so that the fully diluted share count on a non-GAAP measure is 48.6 million shares. At the current price of $4.70, the market capitalization is about $228 million.
The Company expects to have cash revenues of $19 to $21 million in 2014, $50 to $53 million of cash R&D expenses and $15 to $17 million of cash S, G& A expenses. This indicates an operating cash burn of $44 to $50 million for the year. The burn in the first quarter was $18 million indicating that the burn for the remainder of the year could be $26 to $32 million. The cash and investment position at the end of 1Q, 2014 was $101 million so that cash at year end 2014 could be $69 to $75 million.
I cannot rule out the possibility that Cytokinetics may need to do an equity financing in 2015. However, milestone payments from Amgen and Astellas may allow the Company to go forward without a financing. It is also possible that CYTK might obtain a partner for tirasemtiv; this would almost certainly rule out an offering.
A Refresher on The ALSFRS-r Scale
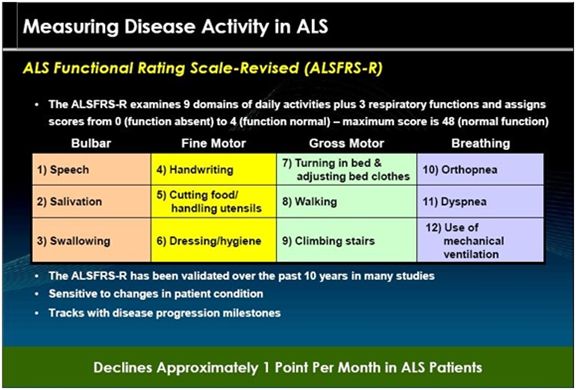
The ALSFRS-r scale has been considered by the FDA and key opinion leaders as the most important measure of the degree of impaired functionality caused by ALS and is used to track the decline in patients. It combines nine criteria involved with daily activities and three involved with respiratory function. It is based upon a subjective measurement by the physician or patient for each function. A score of 0 indicates that there is an absence of the function and a score of 4 means that there is normal functionality. The 12 elements of the scale are shown below:
A normal person would score 48 and a person with ALS would score about 36 a year or so after diagnosis. There is a persistent decline in the ALSFRS-r score of about 0.8 to1.0 points per month. Key opinion leaders state that while there can sometimes be a period of stability of a month or two, there is an inexorable decline and sustained improvements just don’t occur. Death usually occurs when ALSFRS-r reaches 16 to 18. Death is almost always the result of respiratory failure or complications such as infections that can occur when breathing must be assisted by mechanical ventilation.
Positive Results in Two Key Secondary Endpoints Keeps Hopes Alive
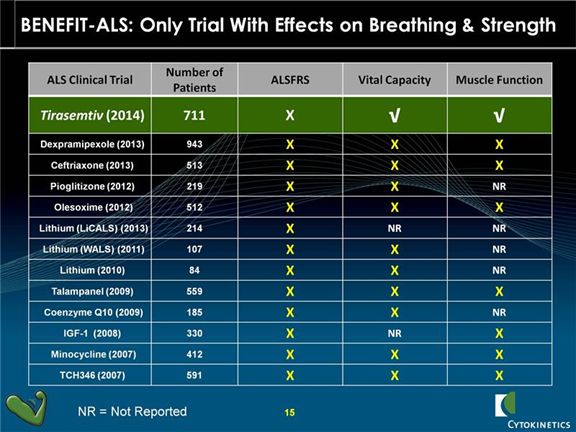
Tirasemtiv joins a long list of drugs that failed to show any improvement in ALSFRS-r and were subsequently abandoned. What differentiates tirasemtiv from these other drugs is that it was the only drug to show an improvement in slow vital capacity and muscle function. The following table shows the drugs that have failed in trials that involved anywhere from 84 to 943 patients.
Slow vital capacity is a measure of the maximum amount of air that a person is capable of slowly expelling from their lungs after taking the deepest breath that they can. Normal adults can exhale between three and five liters of air. SVC is not a measure of how fast, but how completely this slow exhalation can be done. Patients breathe into a commonly used device for measuring pulmonary function called a spirometer. This measures the amount of air exhaled and then uses algorithms based on age, gender, height, weight and ethnicity to measure slow vital capacity relative to what a normal person might do. It reads out a percentage of the level of SVC for a patient as compares to the expected level for a normal person of similar characteristics.
SVC is an indicator of the strength of the skeletal muscles that are responsible for breathing. It is linked to measures of disease progression and is a predictor of survival in ALS patients. It is the respiratory measure that is most commonly associated with death and is used by friends and caregivers to plan for the death of patients. ALS patients know that more than any other thing this measures their disease progression. In the same way that most people know their social security numbers, ALS patients know their slow vital capacity scores; it is measured at every visit. It is also a determinant factor in in the decision to include patients in clinical trials and to determine when there is a need for ventilatory assistance.
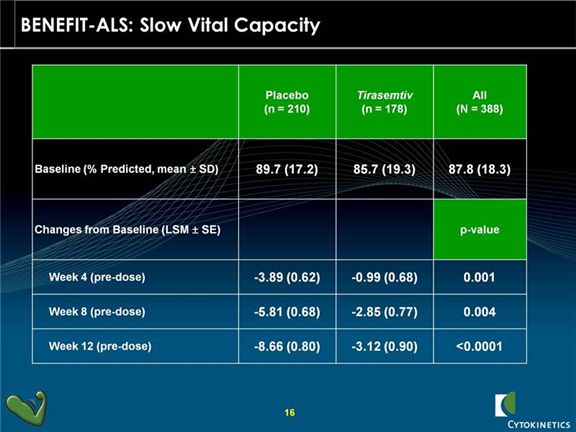
Slow vital capacity was one of the pre-specified secondary endpoints in BENEFIT-ALS and showed a very strong p=0.0006. There was less of a decline in vital capacity for tirasemtiv patients at assessment time points of 4, 8 and 12 weeks indicating a sustained and durable benefit. On an overall basis, the rate of decline was one-third that of control patients.
There was also benefit suggested from another secondary endpoint; Muscle Strength Mega-Score is a measure of strength combining the results from five muscle groups on each side of the body. For this measure, the physician holds a dynamometer in the palm of their hand and the patient pushes back by flexing the elbow, wrist, hip, knee or ankle. It records the force and power that the patient can exert. All of these readings are combined to determine a composite called Muscle Strength Mega-Score.
Tirasemtiv patients showed a slower rate of decline versus placebo patients in the slope of decline of this measure (p=0.016). This was about one third the rate of decline for control patients. While the results over the course of the trial were significant, there were no differences at any time point during the trial that reached statistical significance.
Side Effects Seen in the Trial Caused A Lot of Dropouts
It was known going into the trial that some patients would not be able to tolerate the side effects of tirasemtiv which were largely thought to be dizziness. This was meant to be accounted for by having a lead in period of three weeks in which all patients were given 150 mg of tirasemtiv BID. There were 711 patients enrolled in the trial and 106 dropped out because they couldn’t tolerate the drug leaving 605 patients who were randomized into 302 patients on placebo and 303 on tirasemtiv. In the placebo group 12 dropped out due to side effects and in the tirasemtiv group 78 dropped out early due to side effects.
Adverse events are frequent in ALS but there were more in the tirasemtiv group at 96.7% versus 87.5% in the control group for a difference of 9.2%. There were a large number of measured side effects in which the incidence of AEs was greater for tirasemtiv than placebo: dizziness (31.2%), fatigue (19.0%) nausea (14.1%), confusional state (9.9%), muscle spasm (9.5%), somnolence (9.2%). decreased appetite (6.9%) and insomnia (6.2%). Those side effects that most affected tirasemtiv patients were dizziness (50.8%), fatigue (33.2%) and nausea (21.9%). Looking at all of these numbers, it is obvious that tirasemtiv is a very difficult drug for ALS patients to tolerate.
Views of Physicians Whose Clinical Centers Participated in BENEFIT-ALS
Three key opinion leaders spoke on the April 30 call. Dr. Jeremy M. Shefner, Professor and Chair, Department of Neurology at the Upstate Medical University, State University of New York was the Principal Investigator of BENEFIT-ALS. Dr. Robert G. Miller, Clinical Professor of Neurology and Neurological Sciences at Stanford University, and Director of the Forbes Norris ALS Research Center at the California Pacific Medical Center; and Dr. Merit E. Cudkowicz, Professor of Neurology, Harvard Medical School and Chief of Neurology Service at Massachusetts General Hospital (MGH) and Director of the ALS Clinic at MGH were physician participants in the trial
These physicians feel that slow vital capacity is the best measure of respiratory function and hence the most important variable in determining whether patients can survive longer on tirasemtiv than placebo. Similarly they felt that Muscle Strength Mega-score was the best measure of improvement in muscle strength and these two variables were the most important factors to consider in judging whether this drug has clear biological activity.
They felt that gastrointestinal side effects associated with tirasemtiv probably affected the emotional and physical state of ALS patients so that it had a negative effect on the ALSFRS-r scale which measures a wide variety of activities that are important to quality of life. They also felt that two other secondary endpoints measured in the trial, SNIP and MVV, which did not reach statistical significance, were less important factors for them. In their opinions (it was unanimous), the improvement in SVC in particular and Muscle Strength Mega-score trumped all other measures in the trial.
They unanimously felt that this drug has clear biological activity and the ability to help their patients. They want to see the Company do another trial(s). The primary endpoint of a future phase 3 trial is likely to be SVC with a potential co-primary endpoint of Muscle Strength Mega-score, not ALSFRS-r. I have included comments made by the three physicians who participated in the trial that give an insight into their thought processes.
Dr. Miller
His ALS patients live on average two to five years after diagnosis. The ALS community has seen nothing but trial failures over the 20 years since Riluzole was approved. Riluzole improved overall survival by 2 to 5 months in its phase 3 trials, but had no effect on a scale that was a forerunner to ALSFRS-r, had no effect on breathing and had no effect on muscle strength.
Physicians aren’t sure as to why Riluzole produces an effect; they don’t know the mechanism of action. With tirasemtiv, they see clear effect on improving respiration and strengthening limb muscles. This is as expected given that its mechanism of action is to improve muscle contractility.
In BENEFIT-ALS, tolerability of GI side effects which contributed to weight loss was a key issue. This obscured the ALSFRS-r results as it is well understood that patients who suffer weight loss perform more poorly on this scale as this affects quality of life.
No other drug ever tested has shown an improvement on the scale of what was seen with tirasemtiv in BENEFIT-ALS. It improves muscle strength and breathing. It is a very positive drug despite the weight loss issues that have dragged down results.
MVV as a secondary endpoint is new to the ALS community. It is a hard test to administer and interpret. The relationship of SNIP to disease is less clear than SVC. On the other hand, SVC is well established as a measure of breathing function and predictor of survival.
In the earlier phase 2a studies, he had two patients who showed dramatic improvement in muscle strength during the three week treatment period. One patient could not raise her arms from her side, but after tirasemtiv was able raise her hand. Another wheelchair bound patient was able to improve from a weak to a very strong handshake. He took this as an indication that tirasemtiv has a rapid and clear biological effect in some patients.
His patients who participated in BENEFIT-ALS would clearly participate in a new phase 3 trial. His patients were disappointed that they could not continue in the trial.
He believes that further studies should be done. He feels that SVC and Muscle Mega-strength are very important endpoints with SVC being a predictor of survival. SVC could be the primary endpoint for a new phase 3 trial or potentially there could be a co-primary endpoint of SVC and muscle strength Mega-score.
Dr. Cudkowicz
This drug improves muscle strength. No other drug has ever done this.
Patients want access to drugs that may help them and will tolerate side effects if they think tirasemtiv will improve breathing and muscle strength.
The side effects with tirasemtiv are manageable. Patients will accept the side effect risks of tirasemtiv. Also, the side effects can be managed.
Just getting a few months more of life with some quality is meaningful to ALS patients.
She is excited about what has been learned with tirasemtiv and wants to move the drug forward to regulatory approval.
She is not too concerned about the failure to reach significance in the ALSFRS-r scale. This is a broad and crude scale.
Her patients would be eager to participate in a new phase 3 trial.
Dr. Schefner
This was a positive study and there should be a new study undertaken. Physicians can make the drug more tolerable through proper management of side effects and through better dose titration.
The endpoint of a potential phase 3 trial would have to be discussed with the FDA. However, he feels that the FDA is not set on ALSFRS-r as an endpoint and might accept SVC and Muscle Strength Mega-score as primary endpoints in a new phase 3.
The GI side effects were a surprise. Investigators knew that dizziness was an issue, but in the three week phase 2a trials, they had not seen the problem with GI side effects that materialized during BENEFIT-ALS. The GI side effects are slower to materialize and are difficult to sort out from the many other ailments of ALS patients.
Tagged as ALS, BENEFIT-ALS, cytk, Cytokinetics + Categorized as Company Reports





Larry:
I was thinking: “still too iffy for a speculative investment” for the majority of your extensive report… then at last I came upon what seemed the real passion of these 3 opinion leaders… (of course, I don’t know if they let emotions cloud their judgments: nobody likes to see their patients die, and also they don’t have to pay for a new and expensive trial themselves, so without any costs to them, why not?)
So here’s my question: can and will these doctors lobby the FDA to allow the primary endpoint of another proposed trial be the slowed decline of SVC and muscular strength instead of ALSFRS-r?
And if they do, how influential could they possibly be? I mean, is this ever even done? Thanks. -L.
First of all, the FDA is made up of very competent people with an impossible task. If they approve a drug and it goes on to be a major clinical success nobody says thanks FDA. However, it they approve a drug and it is found to have unexpected, troublesome side effects they are excoriated by the media and called before Congress. With these rules of the game I would be cautious and so would you and so is the FDA.
However, ALS is a dreadful disease with no therapy and therefore no hope once a diagnosis is made. I think the FDA would be more aggressive on tirasemtiv and might agree to a primary endpoint of SVC. However, they haven’t called me yet for my opinion.
So, on point you’re saying that the FDA is so competent that no one lobbies?
There’s no amicus briefs or anything of that nature?
All organizations and the people within them have flaws and the appointed leadership of FDA is political. However, but there are people like Bob Temple, Deputy Center Director for Clinical Science and also Acting Deputy Director of the Office of Drug Evaluation, who are exceptional public servants. He has helped many companies establish the evidence needed to gain drug approval.
You must be a lawyer.
Excellent report.