Discovery Laboratories: An Update on the Surfaxin Launch and the Phase 2 Clinical Trial Program for Aerosurf (DSCO, Buy, $1.72)
Introduction and Overview
This note updates recent progress of the company along with my detailed investment outlook which I break into four different stages of time. The launch of Surfaxin has begun and initial results were below expectations causing the Company to withdraw guidance of $8 to $10 million of sales in 2014. New guidance will probably be issued in the summer. The consensus analyst view is for $2 million of sales in 2014. Previous long term guidance was that Surfaxin would reach $40 million of sales within four years and could reach peak sales of $100 million; this might be lowered.
Aerosurf is the blockbuster component of the DSCO story; the phase 2a trial started in February 2014 and I expect topline results in July or August. This is a trial whose primary endpoint is safety which is largely determined by the ability of a baby to breathe. Management has told investors not to expect any indications of efficacy, but the measures used to determine breathing function for safety reasons could give some insight into efficacy.
Success in the phase 2a trial will allow the start of the phase 2b proof of concept trial. The key to success in phase 2b is being able to deliver therapeutic amounts of lucinactant to the lungs. Because this is the active ingredient in Surfaxin, we already know that the product is safe and effective. Success in the phase 2b trial will give investors a very high level of confidence that the phase 3 trials will be successful. I expect the phase 2b trial to read out topline results in late 2015.
The degree of success of the Surfaxin launch is important to the company. However, it is not a gating factor for Aerosurf. Whether its sales in four years are $40 million or more or less won’t affect the potential sales for Aerosurf. I see the addressable market for Aerosurf as $600 million to $1.2 billion in the US and comparable levels in international markets. I do not see competition on the horizon and I expect a very high level of penetration of the addressable markets if Aerosurf is successful in its clinical trials.
I continue to recommend purchase of Discovery Laboratories. Biotechnology investing is a test of will. There are constant surprises on clinical trial outcomes, regulatory decisions, commercialization, patent challenges, capital raising and a host of lesser issues. It comes as no surprise that stock prices are extremely volatile as investors in Discovery can well attest. Investors have to constantly ask themselves why am I investing in this stock and when negative surprises occur (they always do), is that reason still valid. In the case of Discovery, the reason underlying my owning and recommending the stock is that I believe that Aerosurf could lead to a paradigm shift in the practice on of neonatology.
Historical Perspective on the Use of Surfactants in Pre-mature Infants
Before 1990, no surfactants were available to treat pre-mature infants suffering respiratory distress syndrome (RDS) that was caused by a deficiency of surfactant in their lungs. The standard of care was to intubate the babies and use mechanical ventilation to help them breathe. The mortality rate of intubated babies was over 40% and those who survived often had lifelong lung dysfunction due to the disease and the trauma caused by intubation and mechanical ventilation.
In the 1990s, animal surfactants were introduced and revolutionized neonatal care. These agents were added to standard of care by pouring the animal-derived surfactant down the intubation tube and into the lungs. This took the place of the absent human surfactant whose function is to allow the lungs to expand and contract more easily. These babies required much less aggressive mechanical ventilation and ventilatory support. This innovation reduced mortality from 40% to 5% and has been one on the most beneficial drug discoveries of the last 25 years.
However, this procedure did not eliminate the need for the endotracheal tube and the use of mechanical ventilation that still could damage the fragile lungs of pre-term babies. This created a dilemma for the neonatologist as they could intubate, mechanical ventilate and deliver surfactant to save the lives of many babies. However, in those who did not need this treatment, the neonatologist could damage the baby’s lungs for life because of the intubation and mechanical ventilation. Because, it is not easy to determine which babies to treat or not treat, the neonatologist has a terrible dilemma.
This dilemma led to a new innovation which uses an alternate ventilatory support method called nasal continuous positive airway pressure (nCPAP). This does not require intubating the baby with an endotracheal tube or the use of a mechanical ventilator. Instead the support is delivered through nasal prongs positioned in the baby’s nose in much the way that oxygen is delivered to adults. Because this is so non-invasive, nCPAP is extremely popular with neonatologists and almost all babies born pre-term are initially given nCPAP. However, despite its advantages, nCPAP does not provide surfactant to these infants, and thus does not address the fundamental cause of RDS. Unfortunately, as a result, anywhere from a third to as many of two-thirds of these infants ultimately require endotracheal intubation, mechanical ventilation and surfactant administration.
Well-designed studies have shown that for those babies who need it that early surfactant therapy is superior in improving the baby’s outcome as opposed to first putting the baby on nCPAP and then later moving to invasive surfactant therapy via intubation. So when a baby is placed on a nasal CPAP without the benefit of surfactant therapy and then subsequently requires intubation and mechanical ventilation, not only is the active intubation and mechanical ventilation potentially causing damage to the baby’s lungs but also the lateness of the surfactant therapy is further disadvantaging these babies. This has created a dilemma that is created for the neonatologist and is the essence for why Discovery Labs is developing Aerosurf®.
Rationale for Aerosurf
The Aerosurf delivery system is designed to integrate into the CPAP system to deliver aerosolized lucinactant (the active ingredient in Surfaxin® -lucinactant-) to babies receiving nasal CPAP. It also takes into account the baby’s pattern of breathing and their shallow breath, known as tidal breathing. These are very important considerations that have to be accounted for in order to optimize the delivery of the aerosolized surfactant into the lungs of newborns. As important as lucinactant is the highly specialized aerosol generator that was built specifically to deliver Aerosurf.
Aerosol generators have been in use for over 50 years for delivering drugs suspended in a liquid, but lucinactant is different. It is a suspension of a protein and lipids that acts very differently when aerosolized. The leading aerosol generator on the market, the vibrating mesh nebulizer, is only able to aerosolize small amounts of surfactant for a short period of time and then quickly clogs up because of the viscosity of the product. A different and more advanced technology was needed for Aerosurf
Discovery is using a device called the capillary aerosol generator. Interestingly, this product was originally developed by Phillip Morris in an attempt to find a way of delivering nicotine to the lungs without the carcinogens that arise from smoking. That company spent many years and millions of dollars on its development.
When DSCO conducted a detailed search in the early 2000s for an aerosol generator that was better than existing devices, they determined that the capillary aerosol generator was the best available and obtained a license from Phillip Morris. It will be somewhat ironic if one of the major advances in the protection of baby and ultimately adult lungs was made possible by a tobacco company. However, the takeaway message is that this device was developed by a company with very deep pockets and capabilities that DSCO would have had extreme difficulty in duplicating. Over the last seven years from the licensing of this technology, DSCO has honed this device to precisely fit the requirements for Aerosurf.
The Investment Thesis in Four Stages
I divide the investment thesis for Discovery Laboratories into four stages. We are currently in the first stage which is the launch of Surfaxin, a synthetic surfactant that will compete with the long established products derived from the lungs of pigs and cattle. These animal based products are quite effective and are critical in the treatment of pre-mature babies suffering from respiratory distress syndrome. However, animal based products when faced with competition from products more closely approximating human substances generally are phased out. This was prominently the case for insulin. Importantly, retrospective analysis of the phase 3 trial of Surfaxin showed a statistically significant improvement in overall survival over the animal products.
Surfaxin is a stepping stone to a paradigm shifting product that is Aerosurf. Surfaxin and the animal surfactants are administered as liquids through an endotracheal tube into the lungs and are used in conjunction with mechanical ventilation to help babies’ lungs expand and take in more oxygen. Intubation and mechanical ventilation are highly invasive procedures that, while life saving, can cause temporary or permanent damage to the lungs. The need to give a surfactant to a premature baby is not always easily determined. This presents a dilemma for neonatologist. If they delay giving a surfactant to a baby that needs it, the baby’s life can be put at risk. However, the neonatal healthcare community would like to avoid unnecessary intubation of a child whenever possible.
Aerosurf is intended to solve this dilemma. It is an aerosolized dosage form of Surfaxin that can be given through nasal continuous positive airway pressure or nCPAP. The successful development of Aerosurf would solve the neonatologist’s dilemma. Aerosurf has the potential to replace much of the use of Surfaxin and the animal surfactants and to also significantly expand the number of babies who are treated.
Stage 2 of the investment thesis is the conduct of phase 2a and 2b clinical trials with Aerosurf. A phase 2a trial to evaluate safety and tolerability began in February 2014 and topline data should be available in the third quarter of 2014. The study is intended to evaluate safety and tolerability and is not specifically designed to look at efficacy, but there is the potential to get some insight into effectiveness that could have an impact on the stock. However, the primary purpose is to set the stage for the phase 2b proof of concept trial that will start late in 2014 and should read out topline data in late 2015.
It is this phase 2b study that has the potential, if successful, to cause a dramatic move in the stock. Investors would likely view Aerosurf as a potential blockbuster. The phase 3 trials that would then be conducted to confirm the findings of phase 2b would have a very high probability of success. Aerosurf is based on the same active ingredient as Surfaxin, which we know is safe and effective. This takes away the key risks that often cause phase 3 failures. The only question for Aerosurf is whether it can deliver therapeutically effective levels to the lungs and phase 2b should pretty much determine that.
The third stage is the completion of phase 3 trials, regulatory filings and partnering Aerosurf for foreign markets and perhaps some worldwide non-neonatology indications. The partnering will be necessary to fund some large clinical trials in adult and pediatric respiratory conditions such as COPD and cystic fibrosis. It might also bring in enough funds so that DSCO would not need to issue more shares. Stage 3 starts after phase 2b trial completes in late 2015 or early 2016 and continues into 2018 when Aerosurf could be commercially launched. Partnering will probably take place in 2016 after the completion of phase 2b although it could happen earlier.
Stage 4 would be the commercialization of a unique, blockbuster product in Aerosurf for which there is a great unmet need and no competition on the horizon. It also incorporates a unique pipeline opportunity that could be greater than the opportunity of Aerosurf in neonatology. Stage 4 takes place over the 2018 to 2023 period and would be a dramatic period for stock appreciation.
Surfaxin Launch is Slower than Expected
Surfaxin was approved in November of 2013 and 1Q, 2014 was the first quarter of marketing. DSCO has found the going slower than expected. Part of this was underestimating how slow the process of formulary acceptance can be and part may have been due to the bad weather that postponed formulary committee meetings. Sales were de minimus in 1Q, 2014 at $28,000. Another reason for this low level of sales is an accounting requirement that causes companies to under report sales in the early stages of a launch and which I will explain later.
At any rate, DSCO withdrew sales guidance of $8 to $10 million for the first 12 months of marketing which is roughly fiscal 2014. The Company said that it would evaluate the ongoing formulary process and reinstate guidance later this year, perhaps in July or August. This has caused investor concern as to whether the launch will succeed and of course this can only be answered with time. Not surprisingly, this has not gone over well with investors and as it has occurred at the same time as a sharp correction in in small biotechnology stocks, the stock has fallen from around $2.50 earlier in the year to the current price of $1.80.
There are two phases to a launch. During the first part, the Company is trying to get formulary acceptance. Sales may be minimal and investors can only watch the rate of formulary acceptance, but this is not easily extrapolated into future sales. Hence, the formulary acceptance stage can be a period of waiting, frustration and uncertainty that follows on the euphoria of a new product approval. This has led to a common practice by hedge funds of shorting stocks of companies going through the formulary acceptance stage of a launch. The real part of the launch starts after a company has gained critical mass in terms of formulary acceptance.
I think that Surfaxin could provide the basis for an interesting specialty pharmaceutical company in the unhappy event that Aerosurf development fails. In that event and if Surfaxin indeed has the potential to reach peak reach $40 million of sales in four years and $100 million at its peak, DSCO would likely stop all research efforts and focus on becoming a specialty pharmaceutical company. With its proprietary sales force, it could be an attractive marketing partner and gain access to other products through licensing or outright acquisitions. This is a well proven model.
Aerosurf is the Key
Surfaxin could be the basis for an interesting specialty pharmaceutical company, but the focus on the Company and the asymmetric upside potential for the stock comes from Aerosurf. Let me give you a brief reason as to why I believe this is the case. The currently marketed animal surfactants and Surfaxin are given as liquid instillates that are administered by an endotracheal tube (intubation) directly into the lungs of a premature infant with respiratory distress syndrome or RDS. Through lessening surface tension, they make it easier for a baby’s lungs to expand and contract when used in conjunction with a mechanical ventilator. Because of the considerable risks associated with intubation and mechanical ventilators, doctors will only give this treatment to babies with the most severe form of RDS; there are about 50,000 such babies treated each year in this way. A photo of an intubated baby is shown below.
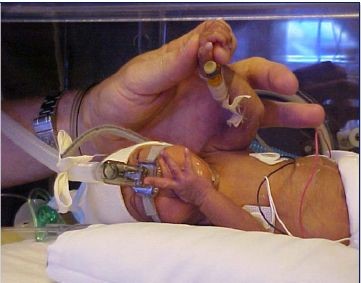
There are an additional 120,000 premature babies born each year who have less severe forms of RDS. These babies are given continuous positive airway pressure through nasal prongs or nCPAP to help breathing. This will help the breathing in and out of these babies. However, about 45,000 babies fail nCPAP and have to be placed on surfactants and mechanical ventilation.
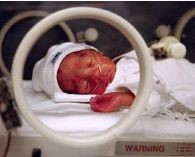
The therapeutic goal of Aerosurf is to develop an aerosolized dosage form of Surfaxin that can be given by nCPAP. Discovery believes that the addition of a surfactant to nCPAP will prevent a significant percentage of these babies from failing nCPAP and moving to liquid surfactants plus intubation and mechanical ventilation. Because there is no way of predicting which of the 120,000 babies treated with nCPAP might progress to mechanical ventilation, Aerosurf potentially would be used in most of these patients. A photo of baby on nCPAP and is not intubated is sown below. Note the use of nasal clips to deliver oxygen as opposed to intubation.
Aerosurf carries a lesser development risk than a drug based on a new molecular entity. The active drug components are the same as Surfaxin, which has been shown to be safe and effective in clinical trials. We know the drug works. Similarly, the manufacturing process has been validated; problems with manufacturing sometimes lead to long delays as was the case with Surfaxin.
The key uncertainty is whether Aerosurf can disperse the active ingredients across the lungs as effectively as the liquid distillate to prevent nCPAP failure. The company licensed in a device called a capillary aerosol generator nearly eight years ago and has refined the device significantly. Studies in the lungs of lambs, which are a good animal model, have shown that Aerosurf can deliver a therapeutically effective dose of aerosolized active ingredients of Surfaxin. If phase 2 and 3 trials substantiate this, Aerosurf has a high probability of being approved.
Aerosurf targets one of the greatest unmet medical needs in neonatology. It also provides very favorable pharmacoeconomics. Use of mechanical ventilation can lead to lung damage that can last a life time and the treatment is very expensive, costing about $2,500 per day. A common complication of mechanical ventilation is bronchopulmonary dysplasia (BPD), which causes serious lung damage and can cost $100,000 to treat. Aerosurf provides enormous value to infants and cost savings to hospitals. I think that it might be priced at $5,000 to $10,000 per course of therapy which for the 120,000 nCPAP treated babies is an addressable market of $600 million to $1.2 billion in the US, with a similarly sized addressable market potential abroad.
The development pathway for Aerosurf is long as I am estimating approval in 1H, 2018. However, I think that the promise of Aerosurf will add consistent value to the stock as the drug progresses through development to commercialization. Discovery intends to commercialize the product on its own in the US and to partner the product for markets outside the US, an event that is likely to occur in 2H, 2015 or 1H, 2016. The Company is not in a hurry to conclude a partnering deal as favorable early results in the phase 2 trials that started in 1Q, 2014 could enhance the value of the deal.
A Closer Look at the Surfaxin Launch: The Role of Formularies
Surfaxin was introduced into trade channels in November of 2013 and 1Q, 2014 was the first full quarter of marketing. The initial focus is on the most influential centers. The Company announced during a conference call on March 15 that they had gained acceptance in five formularies. This was below expectations and as a result the Company withdrew guidance that they would achieve Surfaxin revenues of $8 to $10 million in 2014.
During the May 12, 2014 conference call the Company announced that Surfaxin was now on ten formularies. Management said that they have a very busy formulary committee meeting schedule throughout the rest of the second quarter and expect to see a significant escalation in formulary acceptance. However, it will probably be the end of 2Q, 2014 or possibly 3Q, 2014 before they have a clear read on formulary acceptance and how Surfaxin is being used in the hospital vis a vis the animal surfactants.
The process of gaining formulary acceptance has proven to be slower moving than expected. Management also feels the extreme weather conditions experienced in 1Q, 2014 delayed P&T meetings. It can be the case that just one person being absent can force a rescheduling and this was a challenge in 1Q, 2014.
One of the bright spots was that for each of the first five hospitals that approved access, Surfaxin was added to formulary. Sometimes, a new product is just approved for a trial and its use is restricted. Perhaps only one doctor can prescribe it. The idea is to get a sense of the value of the product before adding it to the formulary without restrictions. However, in these five cases because the product is on formulary, Surfaxin can be prescribed by any doctor. If this trend continues, it would be a big plus.
With respect to revenues, formulary acceptance is no guarantee of revenues. However, DSCO is not going see revenues until the hospital places Surfaxin on formulary. The consensus view of analysts is that Surfaxin will achieve approximately $2 million of revenues in 2014.
This P&T committee is made up of multiple constituencies including physicians, pharmacists, hospital administrators and sometimes lay people. Neonatology may not be represented on the committee directly and if this is the case DSCO must find a neonatologist who will champion the product before the committee. The pharmacist plays a central and sometimes gating role on the committee. They report to the hospital administrator who generally evaluates the pharmacist on their ability to hold down costs.
The pharmacist often bases their decision on a new drug primarily on the basis of cost and this creates a disincentive to add new drugs to the formulary. This means that DSCO must separately approach the pharmacist with a pharmacoeconomic argument. The WAC price of Surfaxin is about $860 per vial and usually one and one-half vials are required per administration resulting in a drug cost per treatment of about $1,300; this is about a 15% premium to the animal surfactants.
Surfactants are included in hospital DRGs in which the hospital is reimbursed perhaps $20,000 for the total procedure involved in intubating and mechanically ventilating a pre-mature infant. While Surfaxin doesn’t represent much of a premium over animal based surfactants and its cost is small as part of the DRG, the pharmacist will still pay attention to cost savings that may be possible with the continuing use of animal surfactants.
The P&T committee may not be the end of the process. In some cases, it might still require an additional review by the hospital CEO’s financial team. This is especially the case if it is a single source contract.
Phase 2a Clinical Trial of Aerosurf is Underway
The Aerosurf clinical program will start with a phase 2a study that is primarily intended to evaluate safety and tolerability. They are recruiting pre-mature infants from 29 to 32 weeks gestational age. The trial will enroll 36 patients in an open label randomized trial who will be randomized to nCPAP plus Aerosurf versus nCPAP alone. The trial will be divided into three stages starting with a low dose Aerosurf followed by medium and high dose.
Recruiting patients for neonatology trials can be difficult as parents are naturally reluctant to expose their babies to anything not proven. Parents are told that they can put their babies in a trial in which they will receive either nasal CPAP or they can be put on aerosolized Surfaxin + nCPAP. The trial is open label so that physicians know which babies are on Aerosurf and if there is any issue, they can be moved from nCPAP + Aerosurf to nCPAP. The expectation is that enrollment won’t be difficult.
They will be performing this trial at four sites. These sites were selected on their demonstrated clinical excellence in the use of nCPAP. They also looked for sites that had demonstrated that they were good recruiters and good collaborators in a multi-center trial.
After the first low dosing group is completed, the trial will be paused as an independent Safety Review Committee reviews the safety and tolerability information collected on those treated babies relative to the controlled babies that are receiving nasal CPAP alone. If this safety committee gives the go ahead, they will move on to a second higher dosage of lucinactant.
It is important to understand that the dose is being increased by increasing the duration of the aerosol delivery. They are not manipulating the concentration of the drug or the flow of the drug through the system. This makes titration of dose relatively easy and allows the pattern of the baby’s breathing and the total breath of the baby’s breathing to be taken into account. Again there will be a pause and evaluation for safety and tolerability before moving on to the third dosage group.
The primary goal of this trial is to determine the safety and tolerability of Aerosurf at these dosages. The safety and tolerability when lucinactant is given as Surfaxin through an endotracheal tube has been established, however, the capillary aerosol generator is a different means of administering the product and they have to determine safety and tolerability with this method of administration as well.
There could be some signal of efficacy in the trial. The physicians are looking at how the babies are tolerating Aerosurf and will be looking most closely at the ability of the baby to breathe. In doing so, they will measure such things as oxygen saturation and level of nCPAP support required which are also measures of efficacy. There could be some separation of the drug group from the control group although the trial is not specifically designed to capture this effect. Physicians will also note the need to intubate babies from both groups.
According to ClinTrials.gov, the phase 2a trial started in February 2014. The final data collection for measuring the primary outcome measure is scheduled for completion in June 2014. The study should complete in August 2014. This suggests the release of topline results in July or August.
A successful outcome of this trial would be the demonstration of safety and tolerability in this population. This would allow them to go forward with the phase 2b part of the trial. As was just noted, they may see some signs of efficacy, but this trial is not designed to demonstrate efficacy. It will be a major success if safety and tolerability are demonstrated so that they can progress through to phase 2b.
The phase 2b will be performed in the same four centers as well as additional centers. The trial could start in late 2014 or early 2015 and complete in late 2015. This trial will be specifically designed to look for an efficacy signal and to determine the design of the phase 3 trials.
Financial Issues
Discovery recognized $28,000 of Surfaxin revenue in the first quarter. They are using the sell through method for revenue recognition which means sales made to their specialty distributor are deferred until the distributor sells the product through to the hospital and all other revenue recognition criteria have been met.
The operating cash burn in the first quarter was $11 million and the guidance for the second quarter is for $11 million of cash burn. The Company ended 1Q, 2014 with $75 million of cash. No guidance on the burn rate was given for the latter half of 2014 or for 2015. The only guidance was that management believes that it has sufficient cash to see the Company through the release of topline results for the phase 2b trial, which is the latter part of 2015. If the company were to continue to burn cash at $11 million per quarter, it would have $53 million of cash at the end of 2014 and $9 million at the end of 2015. However, the larger phase 2b trial for Aerosurf could increase the quarterly burn in 2015.
The Company has $23 million of cash available through an ATM. It has long term debt of $30 million due to Deerfield which is payable in three $10 million installments beginning in February 2017. However, if certain milestones are met in relation to sales and market capitalization, this debt repayment may be deferred.
As of the end of the first quarter they had approximately 85 million shares outstanding as well as 14.5 million outstanding warrants. Included in the outstanding warrants were 4.6 million with an exercise price of $1.50 and an expiration date of February 2016. There were also 2.1 million options. If all the options and warrants are ultimately exercised there would be 102 million shares outstanding. At the current price of $1.72, the fully diluted market capitalization is $175 million.
Tagged as Aerosurf, Discovery Laboratories Inc, DSCO, respiratory distress syndrome, Surfactant, Windtree Therapeutics + Categorized as Company Reports




