Northwest Biotherapeutics: Promising New Data Was Just Presented on DCVax-L in Recurrent Glioblastoma Multiforme (NWBO, Buy)
Executive Summary
Northwest Biotherapeutics has just presented data on 51 patients whom investigators originally intended to enroll in the ongoing, critically important, 348 patients phase 3 trial of DCVax-L in newly diagnosed glioblastoma multiforme. They became ineligible when their cancer progressed before they could enter the trial. However, they were treated nearly identically to the 348 patients and followed to see how long they survived; the survival data was striking. This increases the expectation for success in the ongoing phase 3 trial.
Glioblastoma multiforme is the most deadly form of brain cancer. If unchecked, it grows rapidly in the narrow confines of the skull and begins to interfere with bodily functions controlled by the brain. In advanced cases it actually begins to push the brain into the brainstem that connects the brain and spine. The current standard of care is based on surgery removing as much as the tumor as possible and then treating with radiation and chemotherapy. In almost all cases, the tumor begins to regrow and when it does clinical studies show that 50% of patients die within about 9 months. There is no approved drug that has shown it can increase survival in these recurrent glioblastoma patients. These 51 patients were primarily this type of desperate patient.
There were 20 of these 51 patients who were classified as rapid progressors. Even within recurrent glioblastoma, these patients are probably the sickest of the sick. Half of these patients treated with DCVax-L lived 15.3 months or more which represents an improvement of about 6 months when compared to median survival seen in historical studies. Generally, a 4 to 5 month improvement in an aggressive cancer like this is considered a major advance.
There was also survival data for another 25 patients who were in a better state than the rapid progressors but still much sicker than the vast majority of GBM patients. Half of these patients lived longer than 21.5 months. To put this in perspective, this is an impressive result even if compared to survival expectations for much healthier newly diagnosed patients whose survival expectation is that half will live more than 15 to 17 months. This is almost as striking as the results seen in the 20 rapid progressors.
Northwest has been completely transparent on the results in this study and has provided charts which show the length of survival and in many cases death for each patient. Obviously, the median survival results are impressive but what is striking to me is the number of durable responses as 30% of these 51 patients were alive at two years. This is the hallmark of other immunotherapy drugs and distinguishes them from chemotherapy. It was a durable effect in about 30% of patients treated with the checkpoint modulators-Bristol-Myer’s Yervoy and Opdivo and Merck’s Keytruda- that sparked such great interest in immuno-oncology. Similarly, the CAR-T immuno-oncology drugs of Juno and Kite have shown spectacular results in refractory cases of B-cell lymphomas and leukemias that don’t respond to other drugs.
I argued in my recent report Immuno-Oncology Promises to be the Next “Big Thing” In Biotechnology
http://smithonstocks.com/immuno-oncology-promises-to-be-the-next-big-thing-in-biotechnology/
that Northwest’s dendritic cell cancer vaccine products should be considered in the same light as checkpoint modulators and CAR-T therapy. This has been a view met with skepticism because Northwest is such a small company and because of the long list of failures for previous technologies that sought to develop cancer vaccines. However, with this data I believe that the Company has a data set that is as compelling in highly aggressive glioblastoma multiforme as the CAR-T products in highly aggressive B-cell lymphomas.
There is one other unique aspect of DCVax-L that must be given greater weight with this new efficacy data and that is safety. Opdivo and Keytruda have side effects that are sometimes as severe as chemotherapy. The CAR-T cells produce cytokine storms that result in even more dangerous side effects that can lead to hospitalization and death. DCVax-L has demonstrated to date an extremely encouraging side effect profile. The major side effects are a mild fever of about 102 degrees Fahrenheit that lasts about two days and can be treated with Tylenol and itching at the site of injection. Drugs must be judged not only by efficacy, but also safety. The safety profile of DCVax-L is an extremely important aspect of the drug.
Purpose and Overview of this Report
Northwest’s Chief Technical Officer Marnix Bosch made an oral presentation of this new DCVax-L clinical data at the Immunotherapy of Cancer Conference (ITOC) in Munich Germany on March 26, 2015. He discussed survival data on 51 patients enrolled in an information arm outside the phase 3 DCVax-L trial. The webcast can be seen on this link.
The abstract of the paper was available about two weeks ago and was quite encouraging although it was brief. I began preparing this report at that time, but I wanted to listen to the presentation before finalizing it. This was fortunate because the Company issued a poster on
March 26th that provided significantly more in-depth data on the 51 patients in the information arm. In a much appreciated display of transparency, they have shown the survival status of all 51 patients
Acronyms Used Frequently in This Report
- Glioblastoma multiforme-GBM
- Newly diagnosed glioblastoma multiforme-ndGBM
- Recurrent glioblastoma multiforme-rGBM
- Standard of care- SOC
- Median overall surgery-OS
- Median progression free survival-PFS
- Relapsed/ refractory B-cell acute lymphocytic leukemia-r/r ALL
- Relapsed/ refractory B-cell non-Hodgkin’s lymphoma- r/r NHL
- Relapsed/ refractory chronic lymphocytic leukemia- r/r CLL
- Complete remission rate-CR
- Objective response rate-OR
Reproduction of the Abstract Presented at ITOC
I have started this report by reproducing the abstract presented at ITOC virtually in its entirety. After that, I included sections which provide background and context for the abstract.
Title of Abstract: Prolonged Survival In Patients With Recurrent Glioblastoma Multiforme Who Are Treated With Tumor Lysate-Pulsed Autologous Dendritic Cells
Authors: Marnix L. Bosch (Northwest Biotherapeutics), Robert Prins (UCLA), Linda Liau (UCLA)
The Abstract
Background: Recurrent Glioblastoma multiforme (rGBM) is a life threatening condition, with a mortality rate approaching 100%. Overall survival (OS) in rGBM patients has not materially changed in the past several decades.
We treated 55 rGBM patients with autologous dendritic cells pulsed with autologous tumor cell lysate (DCVax®-L) in an “Information Arm” outside of our Phase III clinical trial. 51 of these 55 patients were not eligible for the trial because they had actual or apparent early progression (recurrence) at a Baseline Visit at the end of 6 weeks of daily radiotherapy and chemotherapy after surgical resection of their brain tumor. 4 of the patients were not eligible for the trial for other reasons (e.g., insufficient doses of DCVax-L).
These rGBM patients received the same DCVax-L product, on the same treatment schedule, in the same medical centers, in the same time period as the Phase III clinical trial, and the data have been collected and maintained by the same CRO managing the Phase III trial.
Aim: To provide compassionate use treatment and to determine OS
of these rGBM patients treated with DCVax-L.
Methods: Disease progression (recurrence) was determined through MRI imaging at the Baseline Visit and at Month 2. All images were reviewed and analyzed by an independent specialized medical imaging company. Each image was reviewed separately by two independent reviewers, and any material differences were resolved by a third independent reviewer. Reviews were conducted using both RANO and McDonald criteria.
OS data is available for all 51 patients. Baseline and Month 2 images are available so far for 46 of the 51 patients. Based on comparison of the Baseline and Month 2 images, the medical imaging review classified the 46 patients into the following 3 groups. The other 5 patients were unclassified, due to lack of available images.
- 20 Rapid-Progressor Patients: A new lesion ≥ 1 cm or tumor growth ≥25% at Baseline and at Month 2
- 25 Indeterminate Patients: Stable disease, modest progression and/or regression, or measurements still unclear
- 1 Pseudo-Progressor: Month 2 image showed resolution of most of the prior appearance of tumor growth
The following chart shows this categorization of patients.

Analysis of 20 Rapid-Progressors
The results for the rapid progressors are shown in the following waterfall plot. I believe that these patients are probably the sickest of the overall GBM population with extremely poor prognoses. In this group, half of the DCVax-L treated patients lived more than 15.3 months. There do appear to be three patients out of the 20 or 15% that lived two years or more. One patient, who is still alive, has so far survived past 37 months, which is extraordinary.
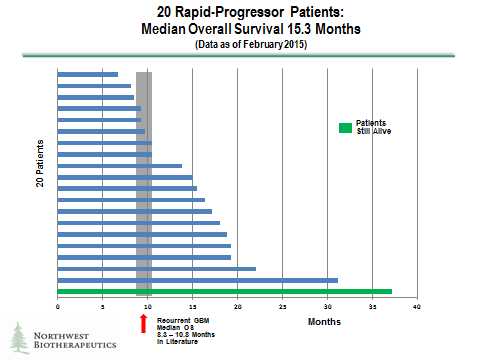
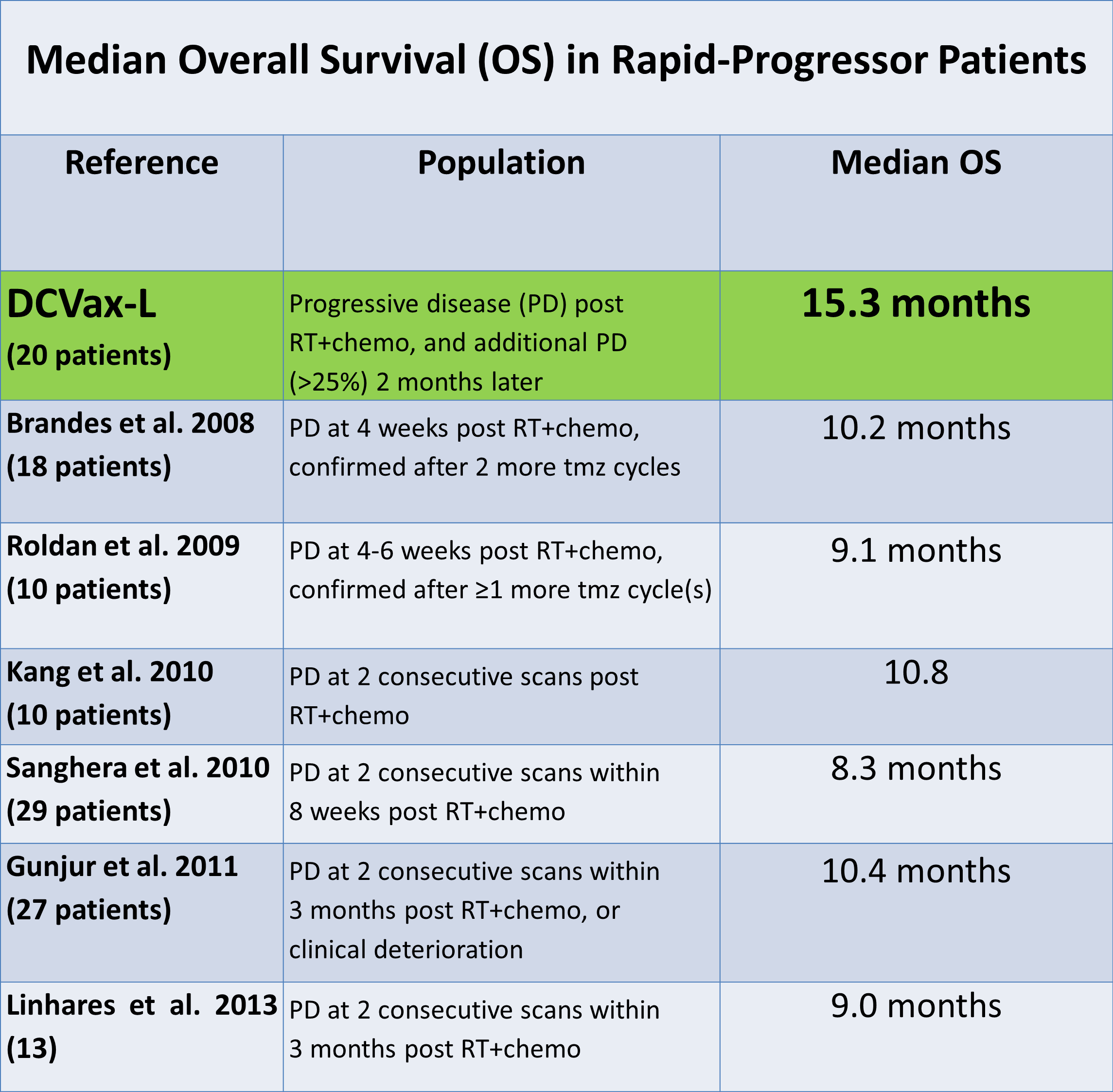
There was obviously no control group for this study so that historical controls were looked to for judging the treatment effect. The authors cited six studies from the literature that studied rapid progressors. The criteria used to classify these patients as rapid progressors was comparable to that used by the independent imaging company and is shown in the following table along with their determination of median overall survival. The range for median overall survival in these six studies of rapid progressors was 8.3 to 10.4 months.
Analysis of 25 Indeterminate Progressors
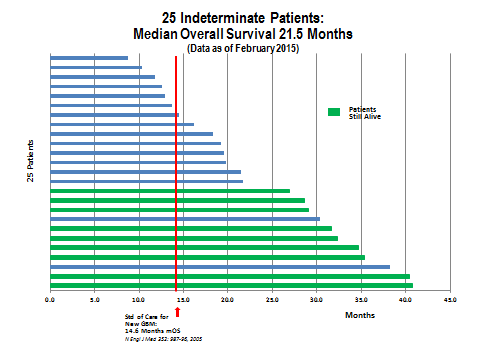
The data for the indeterminate progressors showed that half of the DCVax-L treated patients lived for more than 21.5 months. What is very interesting is that 11 patients survived for 27 months or more and, of these, 9 patients are still alive. This suggests that 44% had a very durable response. Two patients are living at more than 40 months which is again extraordinary. This result replicates what seems to be a pattern with immunotherapy drugs that a sub-group of patients have very long, durable responses. Dare we hope that the two patients past 40 months are cured?
Six Other Patients
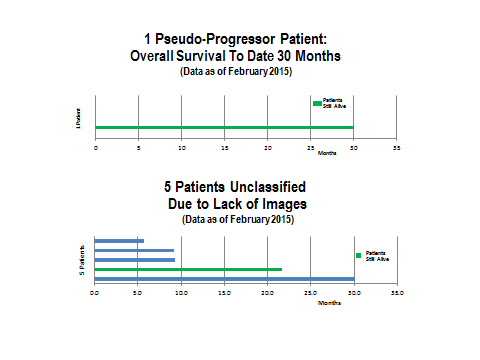
There is not enough information to comment on the one pseudoprogressor other than to say that the patient is alive at 30 months. For the five patients who were unclassified due to lack of images, one is alive at 26 months, one died at 30 months and three died at 5 to 8 months.
Key Results Highlighted by the Authors
The authors summarized the results as follows:
Overall: The median OS is 18.3 months. 15 of the 51 patients who were ineligible for the Phase III trial due to apparent early recurrence lived beyond 2 years, and 12 of the 15 remain alive.
20 Rapid-Progressors: The median OS is 15.3 months (95% Confidence Interval: 10.5 – 17.2) and the range is 6.7 to 37.1 months. A literature search revealed 6 publications with comparable populations of patients which reported median OS of 8.3 to 10.8 months.
12 of the 20 DCVax-L treated Rapid-Progressors lived beyond 13 months; 10 of the 20 DCVax-L treated Rapid-Progressors lived beyond 15 months; and 7 of the 20 DCVax-L treated Rapid Progressors lived beyond 18 months.
25 Indeterminate Patients: The median OS is 21.5 months, and the range is 8.8 to 40.7 months. 9 of these 25 patients remain alive today at more than 24 months, 6 of these 9 patients remain alive at more than 30 months, and 4 of these 9 patients remain alive at 35-40+ months.
1 Pseudo-Progressor: The OS is 30.1 months to date.
5 Unclassified Patients: The median OS is 9.2 months, and the range is 5.7 to 30.1 months.
Conclusions of the Authors
- Patients with evidence of disease recurrence immediately following 6 weeks of daily radiotherapy and chemotherapy after surgical resection of their brain tumor appear to survive longer than would be expected based on data in the literature, when treated with DCVax-L.
- The apparent extended survival of these patients is seen in Rapid Progressor Patients and Indeterminate Patients (as well as the Pseudo-Progressor Patient).
- The combined data suggest a possible survival benefit for patients with recurrent GBM conferred by the DCVax-L treatment.
- The ~30% of survivors who have lived beyond 2 years may reflect long term tumor control.
- DCVax-L treatment (vaccination of patients with autologous dendritic cells loaded with autologous tumor lysate antigens) also continues to have an excellent safety profile.
Separate Survival Data on 8 rGBM Patients Treated at UCLA
The first abstract that was published about two weeks ago discussed a separate group of eight rGBM patients who were treated at UCLA, in prior Phase I trials conducted by UCLA, in which the patients appear to have received fewer vaccine treatments. These patients suffered a recurrence after several cycles of chemotherapy. The poster the Company presented at the ITOC conference omitted discussion of this group, as these patients were not part of the Information Arm. The OS in this UCLA trials group was 13.7 months. The basis for the difference between the UCLA results and the 15.1 month OS seen for the 20 rapid progressors in the 51 patient information arm is unclear. It may be attributable to a difference in the number of treatments.
As a comparator for this group, the authors cited OS results from a study done by Friedman et al. in 2009. This study reported on a comparable group of patients who were treated with Avastin with or without irinotecan. The median overall survival in this group from time of first recurrence was 8.7 to 9.3 months. This suggests an increase in OS in this 8 patient group of about 5.4 to 6.0 months over historical controls.
Providing Clinical and Investment Context on the Abstract
This section of the report summarizes key issues that are important to evaluate results from the informational arm and put them into a clinical and investment perspective.
Design of the phase 3 DCVax-L Trial
Northwest Biotherapeutics is enrolling a 348 patient phase 3 trial of DCVax-L in the most aggressive, fastest growing type of brain cancer, glioblastoma multiforme. The current standard of care (SOC) for this dreadful disease is surgical resection followed by six weeks of daily radiation and the chemotherapy drug temozolomide. After six weeks the patient is treated with six monthly cycles of temozolomide. In this phase 3 trial, at the end of six weeks, the 348 patients are enrolled and randomized; two-thirds receive DCVax-L vaccinations and the others do not.
The primary endpoint of the phase 3 trial is to slow the recurrence or progression of the disease for at least four months. If this endpoint is achieved the trial will be considered a success. Survival is also tracked as a secondary endpoint and will be an important measure of whether the trial is successful. However, when patients who were not initially given DCVax-L see their cancer progress, they will then be given DCVax-L (although they and their treatment will remain blinded). This somewhat confounds the OS analysis.
Importance of progression in glioblastoma multiforme patients
In glioblastoma multiforme the progression or regrowth of the cancer has dread consequences. This is a fast growing cancer that is confined within the skull and as it begins to grow, it quickly begins to interfere with the critical activities that are conducted by the brain. GBM can actually begin to crowd the brain out of the skull and down the brainstem. This is why perhaps more than is the case with any other cancer that delaying progression of the disease is so clinically significant.
There is obviously a close correlation between progression of glioblastoma and death which has been demonstrated repeatedly. The Stupp trial was a 548 patient trial that defined the current standard of care (SOC) as was described previously. Median progression free survival (PFS) is the time at which 50% of patients have the tumor regrow and median overall survival (OS) is the time at which 50% of patients have died. In the Stupp trial PFS was 6.9 months and OS was 14.6 months. The difference between PFS and OS was 7.7 months. This means that half of patients died 7.7 months after the cancer began to regrow or progress.
Roche more recently ran two large trials in which they attempted to show that Avastin added to SOC would improve PFS and OS. They both failed to show that Avastin provided any statistically significant OS benefit but they gave added insight into the relationship between PFS and OS. In the control group of one 978 patient trial, PFS was 6.1 and OS was 15.7 for a difference of 9.6 months from PFS to OS. In a second 637 patient trial, PFS was 7.3 months and OS was 9.4 months for a difference of 9.4 months.
The clear result of these three large trials is to demonstrate that in newly diagnosed glioblastoma multiforme patients, 50% of patients are likely to die 7.7 to 9.6 months after recurrence of the disease. This stage of cancer after progression is referred to as recurrent GBM (rGBM) and the prognosis is much worse than for patients who have not recurred.
The 51 patient information arm of the DCVax-L phase 3 trial
Some of the patients that would have been enrolled in the phase 3 had evidence of tumor progression before they could be entered in the phase 3 trial and consequently were ineligible to enter the trial. Because their tumor had progressed, they would have been counted as failures before they received any dose of DCVax-L. Based on the results of the Stupp trial and the two phase 3 Avastin trials, we might have expected 50% of patients to live about 7.7 to 9.6 months.
There were 51 patients who fell into this category and were considered an information arm outside the phase 3 trial. The (personalized) DCVax-L product had already been prepared and Northwest decided to treat these patients on a compassionate use basis. They were given DCVax-L and managed in the same way as the 348 patients enrolled in the trial. Their results will not be part of the final analysis of the phase 3 trial, but they provide some very important information about how DCVax-L performs in rGBM patients who are much sicker than the ndGBM patients in phase 3.
Rapid progressors experience quick recurrence of GBM
Judgment of tumor progression in GBM patients is made on the basis of analysis of images of the tumors obtained over time through a series of MRI or CT images which are made about every two to three months. In the case of the 51 patients in the informational arm and all 348 patients in the phase 3, these images were read and assessed at a central laboratory. This is critical as assessments by investigators at the clinical sites could vary significantly from site to site and might be more subject to bias.
An image of the tumor is taken immediately after surgical resection at around seven or eight weeks later; this is after a week or two of healing after radiation therapy has ended and before DCVax-L therapy is started. This was followed by subsequent images two months later. Patients were excluded from the DCVax-L trial if the tumor has already begun to regrow in the approximate seven or eight week period since the surgery. Such patients may have a more aggressive form of GBM and are known as rapid progressors; they have a much worse prognosis than other patients.
How do you distinguish rapid progressors from pseudoprogressors?
Determining rapid progressors would seem to be a straightforward determination based on whether the tumor is getting larger over time, but this is not the case. There are some patients whose images show a larger tumor size and brighter image in early scans who are not actually rapid progressors. They are called pseudoprogressors and the appearance of tumor growth in their images following radiotherapy is a false signal, an artifact. It is actually only inflammation or scarring, not tumor. Over time, their inflammation and scarring will heal and resolve, and the appearance of something resembling tumor growth will disappear. There is no good data on survival expectations for pseudoprogressors, but it is generally believed that they do as well or even better than “regular” GBM patients.
Over a period of 8-12 weeks following the initial imaging, the differentiation of rapid progressors from pseudoprogressors can be more accurately determined as rapid progressors will continue to experience growth and they may form new lesions while pseudoprogressors may see the resolution of the inflammation or scarring that had falsely appeared to be tumor growth. It is generally believed that about 20% of patients who initially seem to have evidence of progression are pseudoprogressors and the remainder are rapid progressors or somewhere in between. There are defined criteria that investigators can use to classify patients as rapid progressors. This was laid out in the abstract.
How many of these patients were rapid progressors?
The assessment of who were the rapid progressors was made by the independent central imaging laboratory that is being used to determine the outcome in the phase 3 trial. As might be expected, there is not a crystal clear line between rapid progressors and pseudoprogressors. The lab determined that using the strictest criteria, 20 patients met the generally accepted definition of rapid progression, one met the criteria for pseudoprogression and 25 were somewhere in between and called indeterminate. Five patients could not be evaluated because of missing scans.
How good were results for the rapid progressors?
There was no control group for the information arm which means that the results have to be compared to historical results in other trials. There are always concerns when this is done that we might not be comparing apples to apples. However, in the case of rapid progression there is a wealth of data on patients with characteristics like those in the information arm and they are all consistent with one another in suggesting that median overall survival for rapid progressors is somewhere between 7.7 and 10.8 months.
Going back to the discussion on the Stupp trial and the two Avastin trials we would expect that the median overall survival for these patients would be 7.7 to 9.6 months after their tumors progressed. In the abstract presented by Northwest at ITOC, the Company also cited six smaller studies of rapid progressors in which median overall survival ranged from 8.3 to 10.8 months.
Key opinion leaders generally consider a 4 to 5 month improvement in OS to be a significant advance in an aggressive cancer such as glioblastoma multiforme, but even a smaller improvement can be clinically meaningful – especially in a cancer such as recurrent GBM, in which no treatment to date has achieved any material extension of survival. I previously discussed the Stupp trial; the addition of temozolomide to surgery and radiation improved median overall survival by only 2.5 months but propelled temozolomide to $1 billion of sales. More recently, Medivation’s Xtandi and Johnson & Johnson’s Zytiga improved median overall survival in metastatic colon cancer (an aggressive cancer) by 4.5 and 4.1 months. Both quickly became billion dollar blockbusters.
Median overall survival improvement for 20 rapid progressors
The median overall survival in Northwest’s Information Arm was 15.3 months in the 20 rapid progressors indicating an improvement in median overall survival of 4.5 to 7.6 months when compared to historical controls. These data are suggestive that DCVax-L is a major advance in recurrent glioblastoma.
Comparison to Celldex’s results for rindopepimut in recurrent glioblastoma
Celldex recently reported results for its cancer vaccine rindopepimut in recurrent glioblastoma. Rindopepimut addresses GBM patients who have the EGFRvIII mutation; this is about 30% of the GBM population. DCVax-L addresses all GBM patients including those with the EGFRvIII mutation.
The criteria for enrollment in the Celldex ReACT study were patients whose GBM had progressed and had not yet received Avastin. Avastin helps treat symptoms of GBM, but has not been shown to have an effect on survival. This is similar to the patients in the Northwest 51 patient information arm. There were 37 patients in the Celldex study arm given rindopepimut plus Avastin and 35 who were given just Avastin and constituted a control group. Interim results showed that the rindopepimut arm demonstrated OS of 12.0 months versus 8.8 months for the control arm for a difference in median overall survival of 3.2 months. This was an interim look and some patients were censored; it is possible that these numbers could change slightly and probably in favor of rindopepimut.
The FDA has also granted rindopepimut breakthrough status which is clear indication that they consider the 3.2 month improvement in OS to be very significant. The news on the rindopepimut results has been followed by the stock price doubling from $14 at the time of the announcement in November to $28 currently. The market capitalization increased over $1 billion on this news.
The control group in the Celldex ReACT study showed OS of 8.8 months. This agrees with the estimated OS range of 7.7 to 10.8 months suggested by the Stupp trial, the two Avastin phase 3 trials and the six smaller trials as was previously discussed. We would all prefer to have results from a controlled trial, but there is a great deal of historical evidence to suggest that using expected OS from historical controls provides a sound basis for evaluating the benefit of DCVax-L.
What about the indeterminate group of 25 patients?
These patients partially met most of the criteria for rapid progressors, but they did not meet the criteria as clearly or as fully as the 20 who were clearly rapid progressors. In this group of 25 indeterminate patients, the OS was 21.5 months; there is no clear peer group to compare them to.
Based on all of the available evidence, rGBM patients have much shorter OS than ndGBM. As was previously discussed the OS is about 7.7 to 10.8 months from the time of progression. Judged against the data for rGBM, the OS of 21.5 months for these 25 patients is impressive representing a 10.7 to 13.8 month improvement in OS.
The data remains impressive when compared to OS from the Stupp trial and the two Avastin phase 3 trials in ndGBM patients who are much healthier and are newly diagnosed with GBM (i.e., at an early stage of the disease). The OS expectation in these Stupp and Avastin trials was 14.6 to 16.7 months. Judged against this, the OS of 21.5 months for these 25 patients in Northwest’s information arm is still impressive, representing a 4.8 to 6.9 month improvement in OS.
What about the one pseudoprogressor?
This patient remains alive at a little over 30 months. There are some studies which indicate that OS in these patients is somewhere between 16 and 26 months, but this is not as well documented as are rapid progressors.
Summary of all published data on DCVax-L
The published data on DCVax-L until now was based on phase 1/2 results in 20 ndGBM patients. The data was spectacular as it showed OS of 36.4 months and PFS of 25.0 months. This compares to 14.6 months and 6.9 months in the Stupps trial that established standard of care.
Generally, key opinion leaders consider a 4 to 5 month improvement in OS in an aggressive cancer like ndGBM to be a major advance. The Phase 1/2 trial data on 20 patients has all the appearance of a home run, but it has been criticized as being an open label, non-randomized trial done at one medical center and with a small number of patients.
This new data on 51 patients in the information arm provides strong support for the phase 1/2 data. It appears that in a much sicker group of rGBM patients (the rapid progressors) there are OS improvements of somewhere between 4.5 and 7.6 months relative to historical controls.
The data base for DCVax-L is now based on 79 patients from three different data sets which all suggest a highly impressive improvement in OS. I think that there may also be 20 or more patients who have been treated with DCVax-L on a compassionate use basis; these patients suffered from a variety of solid tumors. Results for these patients have never been made public, but I am aware based on anecdotal information of two cases of very significant improvement.
Thoughts about the small trial size and non-randomization of the 51 patients
Critics will be quick to point out that we are dealing with a small patient number of 51 patients broken into smaller sub-groups of 20 rapid progressors, 25 indeterminate patients and 1 pseudoprogressor and of course this trial was not randomized. In comparing to the rindopepimut ReACT trial in rGBM which enrolled 37 patients in the treatment arm and 35 in the control arm, the numbers of treated patients is greater for DCVax-L. However, ReACT has a control group and DCVax-L is comparing to historical controls.
However, if you compare the data set of the informational arm to results for the much-ballyhooed CAR-T companies Juno and Kite the comparisons are eye opening. I use the term ballyhooed because Juno has a market capitalization of $4.8 billion and Kite is at $2.6 billion. The disease targets for Juno and Kite are relapsed/refractory B-cell lymphomas while DCVax-L is targeted at GBM. These are very different types of cancers; the only thing they have in common is that they are very aggressive cancers with no good treatment options.
Juno is advancing its lead products into phase 2 trials on the basis of 27 patients who were treated in phase 1 open label, non-randomized trials. Kite is advancing its candidate on the basis of 29 patients who were treated in phase 1 open label, non-randomized trials. Neither Juno nor Kite has reported OS in their trials. They are judging results on the basis of response rates.
I think that the data set supporting DCVax-L is certainly as good as and probably better than the data sets that have caused such great excitement with Kite and Juno.
Issues with Informational Arm Data
As was just discussed at length, this paper suggests that the median overall survival for DCVax-L in rGBM is 5 to 8 months longer than median survival with existing treatments. There is also every reason to hope that the results in newly diagnosed and healthier GBM patients that are the subject of the current phase 3 trial will do better, but this remains to be seen. I do note that the available data on 20 ndGBM patients showed 36 months of OS which is 21 months better than the 14.6 months in the Stupps trial.
There are shortcomings of the data that I will now address. I think that we will see an aggressive push back from bears on the stock. Northwest has been the subject of ongoing nasty short selling attacks. There are 9 million shares short for NWBO and perhaps another 4 million naked shorts. These investors are in a really desperate position and will try to conjure up ways to demean the results. I anticipate what they may focus on in the next sections.
There is no data from randomized trials
The bears will say that the DCVax-L data is based on non-randomized trials which make it much less reliable. It will require a randomized trial to establish that there is a difference between DCVax-L and standard of care in rGBM.
This argument has merit. However, there are six separate studies that suggest that a historical OS is in the range of 8 to 10 months with rGBM. And of course, the rindopepimut study substantiated this as it showed that standard of care produced median overall survival of 8.0 months in the EGFRvIII sub-group. While randomized trial results are more reliable, there is strong evidence that median overall survival in rGBM patients treated with standard of care is in the 8 to 10 months range and DCVax-L is increasing median overall survival in rGBM by 5 to 8 months.
The number of patients is too small to draw conclusions
I agree that we will need to see these results replicated in more patients before we can have strong confidence that DCVax-L is providing a treatment effect. Nevertheless, the body of evidence on data in 79 patients is a very strong signal of activity and is consistently positive.
I would also point out that the ReACT trial of rindopepimut in rGBM had 37 patients on drug and 35 on control. This compares to 59 patients who have been assessed in the informational arm and at UCLA. The number of treated patients is similar, but of course there is no control arm. ReACT was randomized, but its patient numbers are small. I note that despite the small numbers of patients treated with rindopepimut that these results led to a designation from the FDA of breakthrough status. As previously mentioned, the stock price doubled on this news and the market capitalization increased over $1 billion.
The two CAR-T companies, Juno and Kite have been the subject of intense medical interest, and these companies carry multi-billion market capitalizations. Importantly, the data set for both companies includes a small number of patients treated in open label, non-randomized phase 1 trials. In the case of Juno there is only data on 27 patients and for Kite there is data on only 29. Juno has a market capitalization of $4.8 billion and Kite has a market capitalization of $2.6 billion.
Clearly, the market has attributed great value to the Celldex, Juno and Kite data despite the small number of patients treated. In the case of Juno and Kite, investors have not been concerned that the data was created in open label, non-controlled trials. I think that an objective analysis suggests that the Northwest data base is at least as good as that of these three highly regarded companies.
How do we know that pseudoprogressors were not mistaken for rapid progressors?
This argument can’t be summarily rejected. However, the criteria used to determine rapid progressors in the informational arm are currently accepted by the GBM investigator community, and the criteria were applied to NWBO’s patients by an independent company with specialized expertise in making this judgment.
An Overview of the Data Set for the CAR-T Companies
The data sets for Juno and Kite in refractory B-cell lymphomas have caused such great medical and investor excitement. The key elements of these data sets are discussed in the next sections.
Juno
-Cancer Target
Juno is planning on advancing two lead products into phase 2 development this tear; these are JCAR015 and JCAR017. It has decided not to advance a third product JCAR-014 into further clinical trials. Each of these products specifically targets CD19 antigens that are widely expressed on B-cell lymphocytes. The mechanism of action has some similarities to Rituxan, Roche’s very successful monoclonal antibody against CD20 on B-cell lymphocytes. All of the JCAR products essentially eliminate both normal and cancer cells that express CD-19. Rituxan does the same with B-lymphocytes that express CD-20. The JCAR products are just more effective.
-Clinical Trial Plans
Juno has not conducted any clinical trials for its products. All of the data was generated in phase 1 trials at NCI and leading academic centers: Fred Hutchinson Cancer Research Center, the Memorial Sloan Kettering Cancer Center, the Seattle Children’s Research Institute, and the National Cancer Institute.
Juno plans to begin a phase 2 trial of JCAR015 in mid-2015 in r/r ALL. It plans to continue a phase 1/2 trial of JCAR017 in pediatric r/r ALL that was started at an academic center and it is planning a phase 1/2 trial of JCAR017 in adult r/r ALL and r/r NHL in 2015.
By the end of 2015, Juno plans to have begun phase I testing for at least four additional product candidates using CAR technology and a new phase 1 clinical trial for a fifth clinical-stage product candidate using its TCR technology. In each case, the cancer target is a distinct protein like CD19 that is overexpressed on certain cancer cells.
-Clinical Trial Results
JCAR015 achieved an 89% CR rate in 27 evaluable adult patients with r/r ALL. It compared these results to historical complete remissions in a similar population which it believes to be 10%.
JCAR017 has demonstrated in the phase 1 portion of an ongoing phase 1/2 trial an 85% CR rate in 13 evaluable patients with pediatric r/r ALL.
JCAR014 is not being advanced in clinical trials. JCAR014 in a phase 1/2 trial achieved a 100% CR in 11 evaluable r/r ALL patients and a CR of 60% in 10 evaluable r/r NHL patients.
The number of patients treated by all three products [combined] is 61 patients. In the case of the two products that are being advanced into clinical trials, JCAR015 has phase 1 data based on 27 patients and JCAR017 has phase 1 data on 13 patients. Obviously, these were open label controls with no control group.
-Regulatory Plans
Juno believes that the phase 2 trial of JCAR015 could support accelerated U.S. regulatory approval in r/r ALL in 2016. They believe that the phase 2 trial of JCAR017 in adult r/r NHL could result in approval in 2016 or 2017.
-Manufacturing
In the case of living cell therapies, the manufacturing process is the product so that the development of the manufacturing process carries nearly the same importance as the clinical data. Juno did not manufacture any of the products used in the phase 1 trials. This was done at the academic centers and NCI.
-Toxicities
Very importantly, the side effects of the CAR-T products created by cytokine storms are severe and can require hospitalization.
Kite
-Biological Target
KITE’s lead product is KTE-C19. It is a direct competitor of JCAR015 and JCAR017 as it also targets CD 19 on B-cell lymphocytes.
The Company will also begin a large number of phase 1 trials with other products in 2015 and 2016 that target different antigens in both solid and hematological cancers.
-Clinical Trial Plans
The Company has recently enrolled the first patient in a planned 102 patient phase 2 trial in r/r DLBCL, PMBCL and TFL. It will start a phase 2 in r/r mantle cell lymphoma in 1H, 2015 and phase 2 trials in r/r ALL and r/r CLL in 2H, 2015.
-Clinical Trial Results
In trials done at the NCI, there were 17 patients treated with r/r DLBCL and PMBCL. There was a 65% OR and a 35% CR.
In an NCI conducted trial in 7 r/r CLL patients, there was an OR of 86% and a CR of 25%.
In an NCI conducted trial in 5 refractory indolent non-Hodgkin’s lymphoma patients, there was a 100% OR and 25% CR.
In total, 29 patients with refractory B-cell lymphomas had an OR of 76% and a CR of 38%.
-Toxicities
Severe and life threatening toxicities occurred mostly in the first two weeks after cell infusion and generally resolved within three weeks.
-Regulatory Plans
Kite hopes that the data on the phase 2 trial in refractory DLBCL, PMBCL and TFL will be sufficiently compelling to allow for accelerated approval in 2016. They would then initiate a randomized confirmatory study in refractory, aggressive NHL in 2016 in order to fulfill likely post-marketing clinical study requirements and to convert an accelerated approval to regular approval.
-Manufacturing
Like Juno, Kite did not manufacture the drug used in the phase 1 trials. This was done at the NCI. Kite’s contract manufacturer will be using the same manufacturing process as NCI.
Other Upcoming Catalysts for Northwest
The principal purpose of this report is to discuss the new reported data on DCVax-L. However, I did want to highlight a number of potential upcoming catalysts for the stock:
- Northwest may complete the process of negotiating reimbursement with ten hospital groups in Germany in the coming months. This will be followed by actual product sales.
- The Company will begin two phase 2 trials with DCVax Direct in 1H, 2015. There are no approved treatment options in the case of inoperable tumors and the FDA would move swiftly if the data is positive. As in the case of Kite and Juno, these could be the basis of a regulatory submission seeking accelerated approval assuming success. If so, commercialization could begin in 2017.
- An analysis of the full data set for the phase 1 trial of DC Vax Direct should be released in the next several months. Final data on the last patient may be available in April, 2015. This may not allow enough time for an ASCO presentation in June.
- The final part of the UK Promising Innovative Medicine designation should be completed and DCVax-L approved for commercial use in the UK in several months.
- There could be collaboration with one of the checkpoint modulator developers, likely Merck or Bristol-Myers Squibb, to combine their products with DCVax-L or DCVax Direct.
- The first interim analysis that is triggered by 149 events could be completed for the DCVax-L phase 3 trial at any time. I do not expect the trial to be stopped for efficacy. I think that the data monitoring committee will simply recommend that the trial continue.
- I expect topline data on the DCVax-L phase 3 study in 1H, 2016.
- The available stock for shorting has been greatly diminished by the shorting that has taken place and the buying of the stock by strong institutional hands. There are 9 million shares short and as many as 4 million naked shorts. The situation is ripe for a major short squeeze.
Risks with the Stock
I do want to emphasize that there is no certainty in the development of new drugs and there is a reasonable possibility that despite the encouraging news on DCVax-L and DCVax Direct, that both drugs could fail in their clinical trials. Data created so far is encouraging but is not sufficient to assure success in ongoing clinical trials that are necessary for approval and commercialization.
I would advise investors to carefully consider what such a failure would do to their financial status and peace of mind. I have a significant position in Northwest Biotherapeutics and it would be painful if these drugs fail. However, it would not affect my financial or mental status. I would urge other investors to take the same approach. This is a stance that I take with all emerging biotechnology companies in which I invest.
Tagged as Northwest Biotherapeutics Inc. + Categorized as Company Reports
19 Comments
Trackbacks & Pingbacks
-
Northwest Biotherapeutics: Comments on Two AACR Posters (NWBO, $8.62, Buy) | Expert Financial Analysis and Reporting | Smith on Stocks
[…] information that was presented at ITOC in March, My interpretation of the data can be seen at this link. The full poster is as […]
Comment
You must be logged in, or you must subscribe to post a comment.





WOW………..What a great analysis you have put together in record time….Thank you….
I hope eventually it is posted in other places like SA so people can read it and comment on it…
I am sure AF and JF will bash it…..One question to you is my confusion about the German funding system….Yesterday it was reported that the Germans are looking again at DNDN’s treatment and re-considering it for re-imbursement…..So, why do we have to negociate with 10 hospitals if there is one agency that OK’s re-imbursement….Thank you if you ever have the time to answer this little question…..Again, great job….cheers
I’ll take a stab at this: the PEI of Germany is essentially their version of the FDA. The FDA approves therapies for use but does not set reimbursement rates. That job is left to the CMS (Center for Medicare and Medicad Services) and private insurance companies. Germany’s equivalent of CMS is The Sickness Fund. My understanding is there are several sickness funds covering different geographically areas of Germany (much like there are several Medicare administrators in the US). What’s crazy and obviously time-consuming is they not only have to negotiate with each sickness fund separately but also with each medical department (oncology, radiology, NeuroSurg, etc) within the each Sickness fund. That task is beyond mind-boggling. Hope that helps. SR.
Insightful.
Typically great analysis as usual. I really appreciated your explanation of the differences between Pseudo progressors and Rapid progressors, both in general, and as the trial’s criteria defined them.
Also thank you for your examination and comparisons of JUNO and KITE, their teeny, tiny trial enrollments (when compared to DCVax-L), and their corresponding enormous market caps, with NWBO. It’s amazing to me how the market is missing this, but I guess we know who to thank for that.
Regarding the rapid cohort in the information arm, there is one point that perhaps needs to be reemphasized.
That is, the original DCVax-L trials REALLY did not work against rapid progressive disease response after surgery and chemotherapy.
That’s why the results admittedly caught Dr. Bosch “off-guard.” He attributes this strong result improvement to booster shots that are part of the phase III (and info arm) protocol. The earlier trials did not have booster shots.
This appears to bode very well indeed for the phase III trial, because phase III trial enrollees received the same dosing schedule that patients in the info arm received.
Finally, in the past, NWBO was quick to point out they were finding 80% response rates in patients treated with DCVax-L both inside and outside the trial. In one speech, this even appeared to include treatment compassionate cases in places like Israel.
Removal of rapids from the phase III trial should theoretically raise that response rate to 90% and beyond. (And now we know rapids respond as well).
Excellent analysis with detailed , broad , an specific info……you are the go to man !
Thank you. I try to be thorough and balanced. However, every time I write an article like this, I realize how much I don’t know.
Now that the results from Informational Trial are released, the pricing negotiations for HE in Germany should accelerate.
Germany and other countries (not the USA) evaluate drug pricing by (CER – comparative effectiveness research) comparing the effectiveness of benefit to patients vs the existing standard treatment. For example if there is no added benefit, then the health insurance funds reimburse up to std treatment price, with the diff paid by patient if the price of drug is higher). Similarly the level of added benefit determined serves as the basis of negotiation with the health insurance fund. Until now, this may have been the bottleneck on pricing to-date.
Larry, Thanks for the article and excellent information.
If the final Phase 3 results play out as indicated, excellent safety and >80% overall response rate, then NWBO’s valuation should greatly and easily exceed the $2B and $5B valuations of CLDX, KITE, and JUNO today. Direct is additional and has even greater potential: 3rd generation DCVAX, improved over DCVAX-L in the Phase 3, plus the indications for systemic response beyond the initial tumor. I think the tension is building, this time for the shorts. Any of the upcoming catalysts you mention in the article could easily trigger a sustained PPS increase. And as you say, the situation is also ripe for a major short squeeze.
The data in 20 patients in phase 1/2 ndGBM, 51 patients in the information arm and 8 patients with eRGBM at UCLA all point in the same direction of DCvax-L having biological activity and highly meaningful clinical effect. Also, the approval in Germany for early access and the probable approval in UK for early access add validation from two of the best regulatory agencies in the world. So all of this is very positive, but the gods of clinical trials can be cruel. I would advise against victory laps.
Hi Larry,
Thanks for the excellent write-up. I’ve been wondering about the timeline. I seem to remember a (Zack?) report indicating a rather lengthy clinical trial Ph III ending up late 2016/early 2017 with an approval (if all goes well of course) towards the end of 2017.
Looking at clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT00045968, the “Estimated Primary Completion Date” is listed as September 2015 (last updated date: March 6, 2015).
How do you see the timeline? (I’m not going to keep you to this comment a year from now 🙂
Many thanks!
Best regards
Libouban
The phase 3 trial is event driven so that it is hard to pinpoint the time. The interim look could come at anytime and perhaps this could provide an insight. I am guessing at topline results in 1H, 2016.
Thanks for this extensive and very helpful blog.
I also downloaded 6 papers the abstract referenced and did some analyses.
4 of 6 studies used Macdonald criteria for rapid progression assessment. 1 employed RECIST and the most recent one (Linhares et al. 2013) RANO criteria.
This DC-L study also used RANO criteria which are considered better than Macdonald by many people from academics.
The 6 studies had 107 true rapid progressors in total. 27 out 107(25.2%) lived beyond 15 months. 7 out of 107(6.5%) passed 20 months, with only 1(0.9%) greater than 24 months and ZERO beyond 30 months.
11 out of 20(55%) pts in our DC-L study lived beyond 15 months, 3(15%) > 20 months, 2(10%) > 24 months with 1(5%) still living.
Some bears criticized the lack of scans of 5 patients hinting at collusion or pure luck.
I found that lacking image scans is quite common in previous studies.
For example in Linhares et al. 2013
“One hundred seventeen adult patients with newly diag-
nosed glioblastoma were treated between January 2005 and
December 2009 with radiochemotherapy according to the
Stupp protocol. Seventy patients were available for complete
radiological response assessment.”
And in Roldan et al. (2009)
“…. Of the remaining 70
patients who initiated postoperative concurrent treatment, 27
patients did not meet inclusion criteria: 3 did not complete
concurrent treatment, 7 lacked a post-concurrent MRI and 17
patients were excluded because the interval between the end of
the radiation and the MRI was less than three weeks (3 cases) or
more than seven weeks (14 cases). ”
I think for this compassionate use based informational arm, the radiological data is impressively complete. Therefore I believe the chance of pure luck is quite slim.
Thanks as always for your valuable input.
Hi Larry and anyone reading my comments…..
I watched 60 Min. last night and wished our (NWBO) would get some kind of positive
publicity…..What I don’t understand, (cause I am not competent with these test analysis) is because, our results are so favorable in the Compassionate Use cohort, why isn’t the CRO and the DMCB halting the “L” trial early for great results??? Would the results of the Com. Use group be good enough in the eyes of the CRO and DMCB to un-blind that trial and indicate early approval, if it had been a blinded test??? I hope I am getting my point across….If you have an opinion about 1. why we can’t get positive publicity and what it would take, and 2. Is our CU patient information sufficient to un-blind and stop it for efficacy if it were a blinded test??? Thank you in advance if your
happen to read my questions and think they deserve a response….Glad Winter seems to leaving the NE….Cheers
I also watched the “60 Minutes” feature. It discussed treating rGBM at Duke University by injecting the polio virus into the cancer to invoke an immune system response. If I understood it correctly, 11 of 22 patients so treated had survived. At least two were thought to be “cured”. If this procedure actually works, it could threaten the viability of cancer treatment companies such as NWBO, who have developed costly and complicated treatment processes.
They have a dosing issue as there were a number of deaths as they tried to move the dose up. 60 Minutes glossed over this, but it caught my attention. This will not be a trivial issue to resolve. They can’t just go to the low dose and declare all is well and proceed with more extensive trials. They will have to spend a lot of time on undertanding mechanism of action and this will take time. My guess is that it could be several years before we can see more meaningful results that will allow for a comparison of this drug with DCVax-L. My focus is on the phase 3 trial. If it is successful, the stock will have a dramatic response.
April 2nd PR:
http://www.nwbio.com/nw-bio-announces-40-million-woodford-financing/
“Woodford will purchase $40 million of the Company’s common stock at $7.40 per share, for a total of 5,405,405 shares. The purchase will take place in two closings, with $11.5 million closing on or before April 8, and the remaining $28.5 million closing on or before April 30. There are no warrants, pre-emptive rights, or other rights or preferences. There was no investment banker involved in the transaction.”
Quite an endorsement!
Best regards,
Libouban