Neuralstem: A Closer Look at Encouraging Phase 2 Results for NSI-566 Neural Stem Cells in Treating ALS (CUR, Buy, $2.36)
Observations about the Phase 2 Trial Results
I spent the weekend thinking about the data released in the phase 2 trial and chose the word observation very carefully to describe my current thinking. The full results of the study will be published in a peer reviewed journal and/or presented at a major neurological medical conference. Until we see the full data set and the interpretation of that data by investigators, it is hazardous to reach “set in stone” conclusions.
Thoughts on Efficacy Seen with Responders
The efficacy results were impressive as 7 of 15 patients (47%) responded to therapy with response defined as stabilization, slight improvement or slight decline in the ALSFRS-r scale (correlates with quality of life). The time point for evaluation of outcome in phase 2 was nine months. This confirms the five responses seen in 15 patients treated in phase 1. What was so exciting about phase 1 results was that at least 3 of the patients have lived for about three years post surgery with stable ALSFRS-s scores. The overwhelming majority of ALS patients (80% to 90%) dies or suffers sharp deteriorations in the ALSFRS-r score within two to four years of diagnosis
The phase 1 results were extraordinary for these three patients. In addition, in the phase 1 trial, two other patients experienced a very positive outcome in ALSFRS-r. It is important to understand that the first six patients treated in phase 1 were wheelchair bound or atypical patients who would not be expected to respond to surgery and the goal in giving treatment was solely to determine if the treatment was safe. There was one patient who died of a heart attack not related to treatment in the opinion of the investigator. There were three patients with bulbar symptoms, two of whom showed a sharp decline in ALSFRS-s; the lead investigator Eva Feldman said such patients would be excluded from phase 2.
In determining the response rate in phase 1, the numerator of patients who responded favorably is five but what is the denominator? On intent to treat basis, it would be 15 and the response rate would be 30%. If we exclude the three wheelchairs bound patients, the three atypicals, the heart attack victim and the three bulbar patients it would be 100%.
If we now add the results of phase 2 to those of phase 1, we now have 12 responders. On intent to treat basis, the response rate for combined phase 1 and 2 would be 12 out of 30 patients or 40%. Using the exclusion criteria applied to phase 1, the response rate would be 12 out of 20 or 60%. It may or may not be the case that there is a justification to exclude some phase 2 patients when we see the final results.
In the press release on March 12 that reported topline results for the phase 2 trial, three key opinion leaders involved in the trial described the results as positive and supportive of doing a next step phase 2/3 trial. This will be a 50 patient study with a comparator arm and endpoints comparable to phase 2. The Company suggests that this trial may start this summer.
How Can We Judge the Efficacy Results?
There have been no successful trials in ALS against which to compare these results. In my mind, I compare them to those seen in late stage cancer patients who like ALS patients are in a desperate life threatening condition with a short life expectancy and sharply deteriorating quality of life. In such late stage cancer patients an overall response rate of 47% that is durable over a nine month period as seen with this phase 2 ALS trial would be considered very positive.
Let me give you an example. The checkpoint modulators, Opdivo and Keytruda, are showing durable responses of about 30% in patients with late stage melanoma or lung cancer. These drugs are being hailed as breakthroughs in these diseases by both clinicians and investors. I think that we can view NSI-566 neural stem cells in the same light in regard to ALS. With a 47% response rate, this looks like a breakthrough if there is no issue with safety.
In the 47% of patients who responded to treatment we are seeing durable responses over nine months. One of the key things to look for is whether these responses will be maintained over three years or so as was seen with Ted Harrada and two other patients from the phase 1 trial. Nine months of duration of effect is impressive but two years or more would be extraordinary.
At this point, investors and perhaps the Company don’t understand which ALS patients will respond. ALS is a heterogeneous disease and it is very likely that there will be some sub-groups who don’t respond, e.g. bulbar symptom patients. In addition, the mode of action of the NSI-566 stem cells is to nurture neurons in the spinal cord and prevent them from being destroyed by ALS. In order for the NSI-566 stem cells to work, there have to be functioning neurons. ALS patients with less damaged neurons seem most likely to respond, but how do we identify them? Neuralstem has suggested that measurements of muscle strength at baseline such as grip strength may allow for the identification of those most likely to respond, but this remains to be seen.
Thoughts on Non-responders
The results of non-responders seem disappointing. The average ALSFRS-r score at nine months after surgery was 14 after a baseline reading of about 40. Based on the expected deterioration of 1.0 points per month, I would have expected the average score to be 31. Clearly something was going on.
I can offer three possibilities for this result. The first that we have to consider is that this is signal of side effect issues. It can be argued that the cells and/or the surgery actually worsened the ALS. Until we see the full results of the study, we can’t rule out the possibility. However, none of the three KOLs who participated in the study cited a safety issue with the trial. The press release mentioned just one serious adverse event, but attributed it to the surgery and not the cells. The Company is also saying that a phase 2/3 trial may begin this summer. If there were a serious safety signal, I would think they might not be giving this guidance. Most importantly, this was an open label trial in which the results are known in real time to investigators and the FDA. If there was a serious side effect issue with the trial, the FDA almost certainly would have known almost immediately and put a clinical hold on the trial
Another explanation is that the results seen in phase 2 may be attributed to patient selection. From the phase 1 trial we saw that patients with bulbar symptoms did not respond. Given this and the heterogeneity of the ALS population there may be other sub-groups that just don’t respond. We can also surmise from the mechanism of action that this treatment needs functioning neurons to be effective. As most ALS starts in the lower lumbar region and progresses up to the cervical region, we can hypothesize that patients with only lumbar involvement will respond much better than those with cervical involvement. There may be some point of ALS progression upward in the spinal column at which, the treatment begins to lose effectiveness. The investigators may have been pushing to find this point and treated patients who may be excluded from future trials.
I tend to favor the explanation in the previous paragraph over the side effect concern. However, the disappointing results may be a combination of the two. It might also be a result of small sample size resulting in a poor outcome. Or it could be some other factor that I have not considered.
Investment Thesis
I have a high level of confidence that the efficacy results seen in phase 1 and 2 indicate that the surgically implanted NSI-566 neural stem cells result in a clinically important outcome in some percentage of ALS patients. Based in phase 1 and 2 this could be around half of patients as a guess.
The surprisingly poor results for non-responders in phase 2 was a surprise to me. While I cannot rule out the possibility that this is a safety signal that could potentially be very serious, the arguments that I have offered suggest that the side effect issue will not be a major problem. However, until there is a better understanding of why the non-responders did so poorly, there will be concern on the part of me and other investors. I think that the publishing of the full results in a peer reviewed medical journal in the near future (weeks or a few months) could largely resolve the issue.
I continue with my Buy recommendation on the stock. I think that the sharp decline in the stock on Thursday March 12 was much more excessive than I would have expected based on the data released. I would not have been surprised to see the stock trade up on the news and indeed in the pre-market trading it was up 12% at one time.
At the market opening on March 12, there was some weakness with the stock, but the bottom dropped out after Adam Feuerstein published at 11:26 AM an attack article that he is so known for, that questioned the data. This was followed by a barrage of short selling that led to a close of $2.37 on Thursday March 12; this was down from a close on March 11 of $3.74. I have seen this phenomenon happen over and over on the release of data by small companies even if it is good data. Companies tell me that an alliance of hedge fund short sellers acting in concert methodically take out one bid after another and walk the price down gradually at first and then more dramatically. This price action has the effect of scaring off potential buyers and attracting other short sellers. It is my judgement that these hedge funds can set the stock prices pretty much anywhere they want it in the short term for emerging biotechnology companies like Neuralstem.
The downside risk in the stock that may or may not have resulted from the apparently disappointing results with the non-responders seems to be more than adequately reflected in the stock as a result of the hedge fund assault. We may now have to wait for the final results to be published and for the final design on the upcoming phase 2/3 trial to be announced for confidence to return and the stock to perhaps regain or more likely surpass levels seen on March 11th.In the interim, I would not be surprised to see more short selling pressure from hedge funds.
Risk Factors
While I am very hopeful about the potential for NSI-566, I want to point out that development is still at a very early and high risk stage. Let me point out some things to worry about.
- I am making my judgments on the clinical characteristics of NSI-566 without seeing the total data set from the phase 2 trial.
- Even with full results from phase 2, there may not be sufficient information for me or even key opinion leaders to accurately judge the risk/ reward of the treatment.
- This is allogeneic stem cell therapy. Neuralstem and Stem Cells, Inc. (STEM) are at the forefront and the only companies that I know of that are developing products with this new technology. There is minimal clinical trial data supporting efficacy and safety.
- The poor response in non-responders could signal that the treatment worsens the outcome for some ALS patients.
- The Neuralstem cells have only been implanted in a total of 49 patients. This is a small number from which to judge the safety profile.
- The surgery used to implant the cells is complex and could potentially cause serious side effects.
- The Neuralstem cells as used in ALS have only been used to treat 30 ALS patients.
- The dose that will be used in the upcoming trial will use 20 lumbar injections and 20 cervical injections with each injection using 400,000 cells. Only 3 ALS patients have received this dose.
I hope that I have driven home the high risk of this investment situation. However, I believe that we have seen signals of extraordinary efficacy in 12 of 23 ALS patients treated. Note that I am leaving out six patients in the phase 1 trial that were at the end stage of their disease and had no chance of improvement and the patient who suffered a heart attack.
There are 25,000 ALS patients in the US and there is no effective treatment for them. If NSI-566 can replicate the results of phase 2 with acceptable safety, I think that it has the potential to be a major product. Recently introduced cancer drugs like the checkpoint modulators Opdivo and Keytruda are priced at about $12,500 per months or $150,000 per year. If the surgery were priced at the same price, every 1,000 patients treated in the US would result in $150 million. Of course, this revenue would be shared with the surgeon.
Background on the Course of the ALS
In order to interpret the results of the phase 2 trial, it is important to understand the characteristics of ALS and its course following diagnosis. The reasons why patients develop ALS are not well understood. Perhaps 10% of cases are due to genetic predisposition, but are probably myriad other reasons. It destroys neurons in the spinal cord that control muscle function. Usually but not always, the disease starts in the lumbar or lower region of the spine. Neurons in that region control muscles responsible for controlling the legs, bladder and bowel. This damage ultimately leads to loss of bladder and bowel function and confinement to a wheel chair.
The disease then spreads to the cervical or upper region of the spine. Muscles controlled by cervical neurons control arm and hand movement, speech, swallowing and breathing. Loss of function of cervical neurons has a much more profound effect on quality of life as the patient requires greater caregiver support to eat and dress. Most ALS patients ultimately progress to mechanical ventilation and die of respiratory failure.
The ALS Association estimates that most ALS patients die within two to five years of diagnosis. There are some cases in which the disease has spontaneously remitted, but these are extremely rare. The ALS Association states that 20% of patients live five years, 10% live ten and 5% live 20. In these patients, the disease seems to burn itself out and progression is much slower. These patients are called atypicals; Steven Hawking, the famous British physicist is the most famous atypical ALS patient. Key opinion leaders I have spoken with think that it is more like 5% to 10% of the patients survive for five years. I have found no clinical data that gives an insight into the five year survival rate.
For the vast majority of ALS patients (whether it is 80% or 95%), the course of disease is fast and inexorable. Neurologists rely on a scale known as ALSFRS-r to track the course of the disease. There is a section explaining ALSFRS-r later in this report and if you are not familiar with this scale, you may want to skip to that section before continuing. There are twelve parts of the scale which relate to life functions that are affected by ALS. A physician evaluating a patient assigns a value of 4 (normal function) to 0 (no function) to each function. A normal person would score 48 (12 x4).
Patients are generally diagnosed with ALS when their ALSFRS-r score is around 40. There have been two recent trials of drugs that treat ALS which give an insight into how rapidly the disease progresses. The Biogen phase 3 trial of dexpramipexole enrolled 940 patients and Cytokinetics’ phase 2b trial tirasemtiv enrolled 605 patients. The primary endpoint of both trials was to show a slowing of disease progression of ALS as measured by the ALSFRS-r scale, not to stabilize or improve the condition of patients. Neither trial was able to show a statistically significant effect in slowing disease progression.
Both of these trials showed that the decline in ALSFRS-r for the approximate 1500 patients treated (drug and control arms) was predictably about 1.0 points per month. I use this expected decline to judge the results of NSI-566 in this report; it is a critical assumption in my analysis. Most patients are diagnosed with ALS when their ALSFRS-r score is about 40. Indeed, this was the baseline score for the 15 patients in the phase 2 trial of NSI-566. Most ALS patients die when their ALSFRS-r score reaches 10 to 20. This would be roughly 20 to 30 months after diagnosis.
Another critical assumption that I make is that the slope of the ALSFRS-r scale shows a steady decline, month over month, for the vast majority of ALS patients. This is consistent with what was seen in the dexpramipexole and tirasemtiv trials. Based on this assumption, I conclude that any results which show an improvement, stabilization or only slight decline in ALSFRS-r are attributable to: (1) the positive effect of the NSI-566 stem cells or (2) that the patient is atypical.
Rilutek is the Only Drug Approved for ALS; There Is No Effective Therapy
There is only one drug approved for the treatment of ALS; Sanofi’s (SNY) Rilutek (riluzole), which was approved in the mid-1990s. In younger populations, it has a modest effect on survival of about two to three months. However clinicians report that it has no effect on quality of life or activities of daily living. Even with these poor or limited product characteristics it is used in 2/3 of ALS patients and is reimbursed by Medicare. There are about 75 centers of excellence in the US that treat about 50% to 60% of the US patient population.
Rilutek was studied in two clinical trials. The endpoint of these trials was the need for tracheostomy or death. Interestingly, it did not achieve statistical significance in the first study on its endpoint based on the log rank test (p=0.12) prospectively defined. In the second study the log rank test p value was 0.076. When both trials were combined, it did achieve statistical significance by the Wilcoxon statistical test (p=0.05) and the FDA approved it on this basis. Rilutek appears to increase median survival by 90 days in younger populations.
The most recent information on Rilutek came from the failed Biogen Idec study of dexapramizole in which Rilutek was used as a control arm. It supported the previous data that shows that Rilutek extends survival by two to three months. The failure of dexapramizole was the latest in a long line of failed drug development attempts in ALS. Despite numerous attempts, over the last 20 years since Rilutek was approved no other drugs for ALS have succeeded in ALS.
What Key Opinion Leaders Concluded about the Phase 2
Before I discuss my interpretation of the phase 2 results, let’s first look at what three pre-eminent ALS researchers said. They were uniformly positive on phase 2 results and supported a next step phase 2/3 trial to better define safety and efficacy.
The lead investigator for the trial was Eva Feldman MD, PhD, Director of the A. Alfred Taubman Medical Research Institute and Director of Research of the ALS Clinic at the University of Michigan Health System; she is an unpaid consultant to Neuralstem. Dr. Feldman is past president of the American Neurological Association and a recognized leader in ALS research. Ms. Feldman was quite positive on the trial stating "We were able to dose up to 16 million cells in 40 injections, which we believe to be the maximum tolerated dose. As in the first trial, the top-line data show disease stabilization in a subgroup of patients. Perhaps equally as important, we believe the top-line data may support a method of differentiating responders from non-responders, which we believe will support our efforts as we move into the next, larger controlled trial expected to begin this summer."
The principal site principal investigator at Emory ALS center where most of the surgeries were done is Jonathan D. Glass, MD, Director of the Emory ALS Center. He said "The top-line data look very positive and encouraging. If this proportion of patients doing well after treatment can be corroborated in future therapeutic trials, it will be better than any response seen in any previous ALS trials."
Another site investigator in the trial was Merit Cudkowicz, MD, Chief of Neurology, Massachusetts General Hospital and Co-Chair of the Northeast ALS Consortium (NEALS). She said “"We were very excited to participate as a site in this clinical trial. We are hopeful with respect to the top-line results and we need to move swiftly and safely forward to confirm the responder effect and identify people who might benefit from this treatment approach."
Review of Results of the Previous Phase 1 Trial
I first became interested in Neuralstem when I saw the results of the phase 1 trial that preceded this phase 2. The phase 1 trial of 15 patients dealt primarily with lumbar injections in the lower spine. A detailed discussion of the design and results of this trial can be seen in my report of April 11, 2014. As explained in that report, three of the first six patients treated were advanced patients who entered the study with ALSFRS-r scores of about 32 who were wheelchair bound. These patients advanced rapidly and died 7, 13 and 20 months after surgery. The other three patients were atypicals. There was little expectation of seeing a therapeutic effect in these six patients because their disease was so advanced. The objective was to show safety.
There were five cohorts of three patients each in the phase 1 trial. The first two cohorts were those advanced patients just described. The fifth and final cohort was comprised of patients suffering from bulbar symptoms that are related to deterioration of nerves in the cervical regions. Patients with bulbar symptoms deteriorate more quickly and the NSI-566 cells had no effect in two patients and equivocal results in a third. The results were sufficiently disappointing that Eva Feldman said that bulbar patients would be excluded from phase 2.
The fourth cohort (patients 10, 11 and 12) was the one which included Ted Harrada. The results in this group have been extraordinary. You can see from the following table that these three patients remained stable at 38, 40 and 43 months as judged by their ALSTRS-r scores. Using the word miraculous is appropriate to describe this outcome.
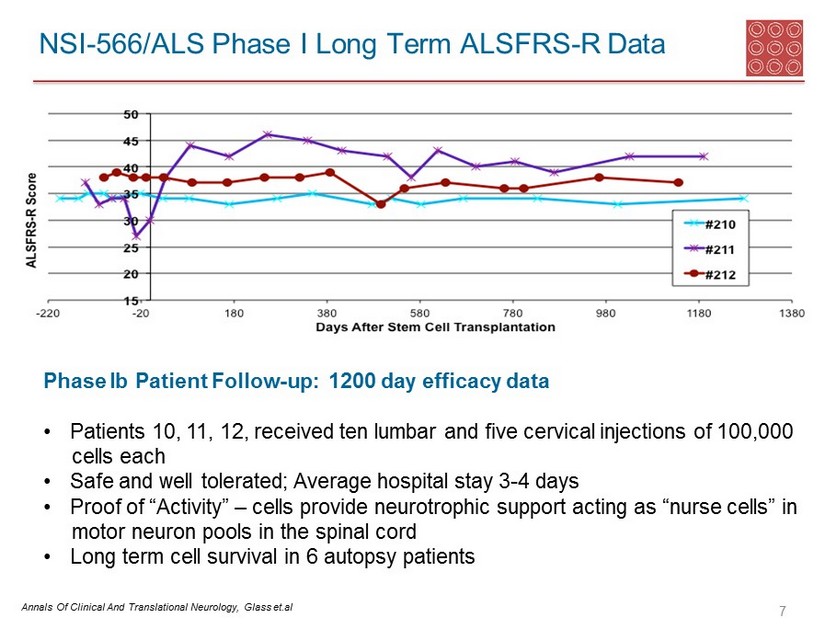
Less attention has been paid to the third cohort of patients 7, 8 and 9, but results in this group have also been impressive. Patient number 9 died of a heart attack that was not attributable to ALS in the opinion of the investigator. While not as impressive as the Harrada cohort, the other two patients do show a significant slowing on the ALSFRS-r scale.
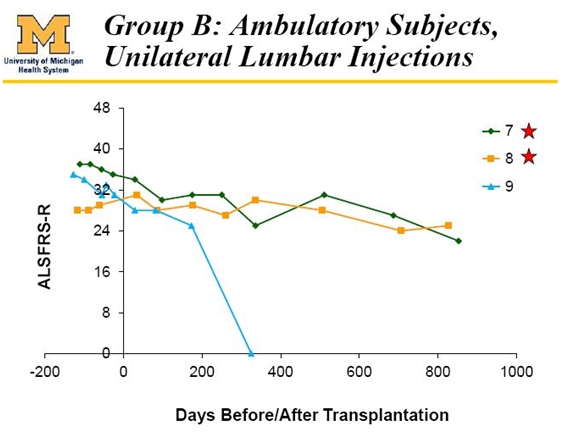
In the five patients that could reasonably have been expected to benefit from the NSI-566 surgeries in phase 1, three experienced an extraordinary result and two experienced a very positive response. At the very worst, the response rate in phase 1 was 5 of 15 (33%), but in my view the response was 5 of 5 or 100% if the six patients of the first two cohorts, the heart attack victim and the bulbar patients are excluded.
It would be very hard to argue that the outcome for all of the five responders can be explained by their being atypical patients. Based on the ALS Association conclusion that about 20% of patients survive five years, we might have expected this type of result in one patient, but not all five.
Topline Data from Phase 2 for 15 ALS Patients
Neuralstem released topline data from the phase 2 trial on March 12 in a press release. This provided only a snapshot of the results in the trial. It stated that 47% of patients in the trial were responders. A responder was defined as a patient who at nine months past surgery, showed either an increase, stability or near zero decline in their ALSFRS-r scores. This indicated that 7 of 15 patients met the criteria for response and 8 did not.
The press release stated that the average ALSFRS-r score at baseline was about 40 for all patients. For the seven responders, the average ALSFRS-r score at nine months was 37 against an expectation of 31 based on historical data. The ALSFRS-r score for non-responders was 14 at nine months versus an expectation of 31. The sharp decline in non-responders is one of the more puzzling aspects of the data.
Remember that a wealth of data from clinical trials suggests that ALSFRS-r score for the vast majority of ALS patients declines by 1.0 points per month. As measured by an average slope of decline of ALSFRS-r, the responders' disease decline was -0.021 points per month, while non-responders' disease progression was 3.0 points per month
Seated Vital Capacity is a surrogate measure of breathing function. This measure showed that responder patients remained within 94% of their starting scores, versus 71% for non-responder patients. Unlike the ALSFRS-r measure, there is not a clinically validated norm to judge the result of this measure. However, it seems to be positive.
The trial met its primary safety endpoints. Both the surgery and cells were well-tolerated. A caveat was that one patient experienced a serious adverse event related to the surgery which was not described.
Design of the Phase 2 Trial
The phase 2 trial enrolled 15 patients who were assigned to five cohorts of three patients each. The patients in the first cohort were patients 301, 302 and 303. Patients in the second cohort were 304, 305 and 306 and so on. The primary endpoint of the study was safety. Key secondary endpoints were ALSFRS-r trends, grip strength and Seated Vital Capacity which were evaluated nine months after the surgery.
The table below shows the breakdown of the number of cells per injection and the number of injections. The first four cohorts were given only cervical injections while the fifth and final cohort was given cervical and lumbar injections.

Looking back at phase 1 trial, there were five cohorts of three patients each who were given lumbar injections with much fewer injections and cells per injection. The fourth cohort was brought back at a later time to be given cervical injections.
My Interpretation of Phase 2 Results
Neuralstem followed up the press release with an 8-K that gave substantially more data on the phase 2 trial. They provided tables that showed individual ALSFRS-r results for both responders and non-responders. These give more of an insight into the results of the trial. The data on the responders was as follows:
Here are key takeaways from my standpoint:
- Seven responses out of 15 patients are impressive. Using the ALS Association estimate that only 20% of patients survive five years, at most we would have seen stability in 3 patients,
- The results were good and confirm the results of phase 1, but were not as striking.
- The responders appeared in each group. There was 1 responder in Cohort A (patients 301-303), 1 in Cohort B, 2 in Cohort C, 2 in Cohort D and 1 in Cohort E. The responders were not concentrated in the later cohorts who received more cells and injections.
- In a homogeneous group of patients, we might have expected that there would be more responses in Cohorts C and D and especially in Cohort E in which there were more cells and more injections.
- I suspect that the spreading of response was probably attributable to the disease status of the patients at the time of surgery. There may have been some patients whose neurons were so damaged that the surgery had no chance of working regardless of the number of cells implanted,
- I think that when the final results are published in a peer reviewed journal that we may get an insight as to whether disease status at entry was a key determinant of response.
The data for the non-responders was shown in the following table.

My key takeaways are as follows:
- At the end of nine months, based on the historically expected decline of 1.0 points per month, we might have expected the average score at the end of nine months would be 31. It was a much worse 14. These patients did more poorly than I would have expected.
- Two of the non-responders were in the final cohort that received the most injections, most cells per injection and was the only group that received lumbar in addition to cervical injections.
- It appears that as many as four patients do not have data at nine months. These are patients 315, 313, 310 and 308. I wonder if these patients died before reaching the nine month period.
- The four patients who did have data at nine months have an ALSFRS-r score of about 20, which is still significantly below what I might have expected.
- The worse than expected data could be explained by three factors: (1) there was a negative effect on these patients as a result of the surgery, (2) the baseline characteristics of these patients in some manner produced poor outcomes, or (3) this is such a small sample that the results occurred by chance.
- As with the responder group, I think that when the final results are published in a peer reviewed journal that we may get an insight as to whether disease status at entry was a key determinant of response.
Identifying Patients who Will Respond to NSI-566
One of the key issues in the potential commercialization of NSI-566 in ALS will be the ability to prospectively define who will benefit. This is a complicated surgery. I think that many or most patients faced with a 50/50 chance of the surgery being able to prolong their life by at least the nine months seen so far, would opt to go ahead. However, it could be a deterrent for some. For that reason, it would be very important if those ALS patients who are most likely to respond could be prospectively identified.
The seven responders all had good grip strength at the time of surgery. The Company mentioned that there may be a correlation between this and response to therapy. They stated that grip strength is a good proxy for how many motor neurons are left in the cervical region where they were implanting the cells. Remember that the hypothesized role of the NSI-566 neural stem cells is to nurture motor neurons that have not been destroyed by ALS. The more neurons that remain, presumably the greater is the potential for benefit.
Neuralstem stated during the Barclay’s health care presentation on March 12 that it believes that grip strength will be a good predictor of who will respond. This could enhance the predictability of outcomes prior to treatment. This will have to be confirmed in the next trial, but management believes that it will be a good predictor and this will be closely studied in the next trial.
Design of the Next Trial
Neuralstem is planning for the next phase 2/3 trial which it expects to begin this summer. It will enroll about 50 patients and the time at which endpoints will be evaluated is 9 months like the phase 2 trial. This is enough time to see a meaningful effect. The primary endpoint will again be safety. As strong as the efficacy data shown in phase 1 and 2 they have only done combined cervical and lumbar injections in only three patents in phase 1 and three in phase 2. Moreover, only three patients in phase 2 have received 20 lumbar and 20 cervical injections of 400,000 cells per injection. The secondary endpoints will again be ALSFRS-r, grip strength and Seated Vital Capacity.
The surgeries will be done at the same three surgeries that participated in the phase 3 trial. These are the University of Michigan, Massachusetts General and Emory. There will probably be one additional center added. Management has not given guidance on how long it will take to enroll the trial. Because three of the centers are very familiar with the surgery given their participation in phase 2, this would encourage quick enrollment. And of course, ALS patients are desperate for any kind of treatment and the potential for perhaps half of them to have nine months to three years stability in their disease should also encourage rapid recruitment.
Just for the sake of illustration, let’s assume that each of the four centers can enroll one patient per month starting on July 1, 2015. This would mean that the last patient would be enrolled in July 2016. There would then be a nine month follow-up on that last patient in March 2017. It might take three months to lock the data base, collect the data and analyze it. Hence, we could be looking at July 2017 for topline results. Management has not given any guidance.
One of the key issues for the next trial is demonstrating an acceptable safety profile. If this is done successfully and the efficacy results are the same as in phase 2 which showed that 47% of patients showed roughly stable disease at 9 months, I believe that the FDA will approve the product. This assumes no limiting safety issues. It would be great if Neuralstem could prospectively determine the most likely responders based on grip strength and other measure, but even without this I think the product would be approved. This would be in 1H, 2018.
Mechanism of Action of NSI-566 Neural Stem Cells
There are only two companies that I am aware of that have been able to produce cell lines of neural stem cells: they are Neuralstem and Stem Cell. Neuralstem is studying these cells in ALS and Stem Cell has begun phase 2 trials in chronic spinal cord injury and advanced macular degeneration. These cells have the same properties as certain stem cells that occur in the central nervous system. Both companies implant these cells with surgical procedures in the grey matter of the spinal cord. I am not aware of any other companies using this approach.
Following surgery, the cells synaptically integrate into the spinal cord. Both animal and human studies indicate that the cells do not migrate more than a few millimeters from the injection site. The surgeries inject the cells into the grey matter of the spinal cord. They appear to surround the motor neurons and release neurotrophic factors that help repair the functioning of motor neurons that have been damaged by ALS and perhaps protect against further injury. They do not appear to grow new neurons so that they must be implanted in a section of the spinal cord in which there are still functioning neurons to be effective.
The effect of the cells appears to depend on the location of the spine in which they are injected. The neurons in lower or lumbar region of the spine control the legs, bowel and bladder. Neurons in the upper or cervical region control the arms and hands, swallowing and breathing. Hence, injections into the lumbar region would be expected to have their primary effect on improving the functioning of the legs, bowel and bladder. Those injected into the cervical region would most affect arm and hand movement, speaking, swallowing and breathing.
A Refresher on the ALSFRS-r Scale
The ALSFRS-r scale has been considered by the FDA and key opinion leaders as the most important measure of the degree of impaired functionality caused by ALS and is used to track the decline in patients. It combines nine criteria involved with daily activities and three involved with respiratory function. It is based upon a subjective measurement by the physician or patient for each function. A score of 0 indicates that there is an absence of the function and a score of 4 means that there is normal functionality. The 12 elements of the scale are shown below:
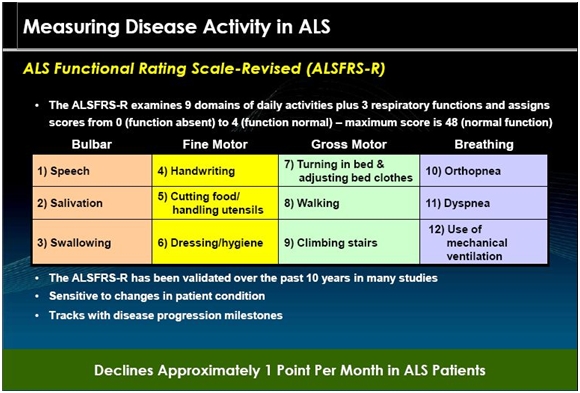
A normal person would score 48 and a person with ALS would score about 36 a year or so after diagnosis. There is a persistent decline in the ALSFRS-r score of about 1.0 points per month. Key opinion leaders state that while there can sometimes be a period of stability of a month or two, there is an inexorable decline and sustained improvements just don’t occur. Death usually occurs when ALSFRS-r reaches 16 to 18. Death is almost always the result of respiratory failure or complications such as infections that can occur when breathing must be assisted by mechanical ventilation.
Tagged as CUR, Inc., Neuralstem, Inc., NSI-566, stem cell treatment of ALS + Categorized as Company Reports
19 Comments
Trackbacks & Pingbacks
-
Comments on Agenus, Neuralstem and Celldex | Expert Financial Analysis and Reporting | Smith on Stocks
[…] of $1.84. I wrote a note on the results on the phase 2 trial that discussed results in depth called Neuralstem: A Closer Look at Encouraging Phase 2 Results for NSI-566 Neural Stem Cells in Treating A… which you may want to refer to.The disturbing information in the phase 2 trial as discussed in […]
-
Neuralstem: Initial Thoughts on Just Released Encouraging Phase 2 Data on the Use of NSI-566 Stem Cells to Treat ALS. Neuralstem Will Proceed to a Potentially Registrational Trial in 2016 (CUR, Buy, $1.50) | Expert Financial Analysis and Reporting | Smith
[…] release was a discussion of the non-responders in the phase 2 trial. See my March 16 report “A Closer Look at Encouraging Phase 2 Results for NSI-566 Neural Stem Cells in Treating ALS for more […]
Comment
You must be logged in, or you must subscribe to post a comment.





In case of further uncertainties regarding 566, do you give much value to Neuralstem’s other pipeline products?
I think that NSI-189 is an extremely interesting drug based on phase 1 data. See this report.
http://smithonstocks.com/neuralstem-phase-1b-results-for-nsi-189-are-very-encouraging-but-it-is-early-days-cur-buy-4-38-for-paid-subscribers/?co=neuralstem
If phase 2 data is promising, it could support much of the current valuation of Neuralstem, but we won’t know this until 2016 or 2017.
WOW…….Larry, your best effort yet……so fair, so detailed, so well organized….Hope to hear more as time goes by…..Cheers
Thank you Smith, I did a similar analysis estimating the -0.007 per day corresponds to a 10 year survival progression including a 1.5year before onset and an average of 40 ALSFRS at that point. After 8.5years the average patient will have an ALSFRs of 18-19 which in my understanding would be likely that the average of the 7 patients would make it to that point.
Then I run over 850 simulation scenarios picking 15 patients where 12.5% have a 10 year expected survival and the result I got is that the chance of randomly getting 7 patients out of 15 is well below 1 in 1000. Thus it is likely the treatment is working for at least some of these 7.
A better identification of the patients in the percentage responders to over 65-70% would definitely make a compelling case for approval in my view.
There is no chance that the responses occurred by chance. Just one response like Ted Harrada’s where he has been stable for a three year period would establish that the cells are effective. It appears that there may be 12 patients with remarable rsponses, but not at the level of Harrada’s. Those ALS patients not given surgery who survive for five years or longer still experience deterioration in ALSFRS-r. They just decline slower and then the disease seems to burn out. These cells are unquestionable effective. A key issue is can we identify those patients who are most likely to respond? This is important but not a show stopper; in any event it looks like they have a good insight into this. The second very important question is whether the poor response in the non-responders is a safety signal which could be a show stopper. I explained in this report why I think this is not the case, but at this point I don’y have data to support that position.
Hi Larry,
Do you know what the design of phase 2 was for secondary endpoint, efficacy? I just wonder how they planned to measure this endpoint from the start. Why they had measure responders vs non responders, with non-responders being worse of then historical control, is still a big question mark for me.
This also bring up the next question: Do we have statistically significant results if we had compared to historical control at 9 months? So since the alsfr score of responders was 37 vs 31, for historical control at 9 months, wouldn’t this have been less dubious, and more meaningfull for the market to compare vs historical control? Now it just looks like they are trying to hide something.
Jerome
The purpose of this trial was importantly aimed at determining if the surgery and cells are safe. The secondary endpoints allowed the company to see signals of efficacy, but was not randomized to a control group. However, the non-responders can be looked at as a surrogate for a control. Comparing to historical control would have to be done for both responders and non-responders. My best guess and at this point it is a guess is that the investigators were trying to look at as broad a range of ALS disease as possible and that the non-responders were so advanced and had such a damaged neuronal status that the implanted cells could not nurture them back to health. This is my read of the body language, but I have no data to base it on. I think the next trial will be centered on ALS patients whose characteristics are like those of the responders.
Hi, Larry
Have you read my second thought on the data in your previous “initial take” blog?
I became more optimistic after spending two days pondering the data.
The problems for me now are why the company presented data like that? Why the company published the data so quickly and provided no data supporting the correlation between grip strength and responders? Why the company didn’t focus on breathing like SVC as all patients got cervical injections? Why not wait one or two months for Dr. Feldman to present all the data and elaborate on her thoughts about responders/non-responders as we already had waited so long?
Maybe I am too paranoid, but the company seemed to invite bear attacks by publishing the results in a rush without thorough and convincing explanations.
All companies that I know of release topline data before final results are published in a peer reviewed journal. I would also suggest that without what I beleive is manipulation of the stock by hedge funds, we would be celebrating the first drug to ever show a stabilization of ALS over a period of none months or more. Don’t underestimate the influence of hedge funds that can cause good news to be viewed as bad news by putting enormous coordinated shorting pressure on a stock.
What if it does turn out that something like grip strength is a solid (even if not perfect) indicator regarding who will respond well, and who will not, something that couldn’t be readily revealed until this trial?
(There were profound challenges that many of the patients in the initial trial were already dealing with that would have masked something as subtle as grip strength. In this trial, they accounted for many of those big and obvious factors with their exclusion/inclusion protocol, and they will further refine those with the next trial, accounting for grip strength, and whatever else they’ve learned about from this trial.)
What if coupled with the impact of (any) surgery on the average ALS patient (see this yahoo thread started by editroid2 on surgery and ALS, http://tinyurl.com/pgetk59), you had several ALS patients with lesser grip strength, indicating greater weakness in their arms and upper body than those with greater grip strength?
Might they have had more challenges in recovering from surgery than ALS patients who had more reserve in their upper body and/or lower body? There are things we take for granted, such simple things as adjusting our position in bed, that might make recovery more challenging for someone with relatively less strength before surgery.
And what if… in accounting for a certain threshold of strength in the next trial, the doctors can help make the difference not only in giving the stem cells more opportunity with more still active nerve cells, but also in screening for patients who have more bodily resources/reserves to call upon as they face the recovery period?
This is an early trial, after all. It makes sense that they would want to learn as much as they can and take what they’ve learned into the next trial.
Linda
What if it does turn out that something like grip strength is a solid (even if not perfect) indicator regarding who will respond well, and who will not, something that couldn’t be readily revealed until this trial?
(There were profound challenges that many of the patients in the initial trial were already dealing with that would have masked something as subtle as grip strength. In this trial, they accounted for many of those big and obvious factors with their exclusion/inclusion protocol, and they will further refine those with the next trial, accounting for grip strength, and whatever else they’ve learned about from this trial.)
What if coupled with the impact of (any) surgery on the average ALS patient (see this yahoo thread started by editroid2 on surgery and ALS, http://tinyurl.com/pgetk59), you had several ALS patients with lesser grip strength, indicating greater weakness in their arms and upper body than those with greater grip strength?
Might they have had more challenges in recovering from surgery than ALS patients who had more reserve in their upper body and/or lower body? There are things we take for granted, such simple things as adjusting our position in bed, that might make recovery more challenging for someone with relatively less strength before surgery.
And what if… in accounting for a certain threshold of strength in the next trial, the doctors can help make the difference not only in giving the stem cells more opportunity with more still active nerve cells, but also in having screened for patients who have more bodily resources/reserves to call upon as they face the recovery period?
This is an early trial, after all. It makes sense that they would want to learn as much as they can and take what they’ve learned into the next trial.
Linda
All good points.
Let me go on to another point. The checkpoint modulators Opdivo and Keytruda have been annointed by investigators, regulators and investors as being a paradigm shifting technology. They are only effective in 30% of late stage melanoma and lung cell cancer patients. At this point, there is uncertainty on how to identify which patients will respond. This is pretty much the case with many or most cancer drugs and many other classes of drugs. Perhaps a 47% response rate is not so bad in ALS if this ultimately proves to be the case.
I recently spoke with an IR guy representing CUR. I called on Friday so was pleased to hear back so quickly. The representative was pretty aggressive in presenting the company’s view and I didn’t learn a lot of new information. One thing I did learn is that the sub 270 timelines were not due to patient deaths, but were due to those patients having July surgeries and their followup visit information hadn’t yet been added to the database.
Hi Larry,
I have 2 questions for you:
The first is about the phase 2b that is about to begin. In you’re analysis you seem optimistic that, if as succesfull as the phase 2a, the 2 b would be enough to file an nda. Others claim that a pivotal phase 3 will be needed. Which would mean that we are 3 to 4 years further away from approval. So is this 2b a phase 3 in disguise, or did they add extra phase 2 before they had the right to proceed to phase 3?
The second is about the graphs from Leuven that Jason posted recently in his analysis. There seem to be about 5 patients out of the 28 that experience for a period of 9 to 12 months a plateau on the alsfrs timescale. Do you have acces to the conclusions of the study? A feel this graph may have been taken out of his context to make a certain point. Also do you care to venture you’re opinion on this in relation to the top line results?
Jerome
Filing off of the upcoming phase 2 data would be a high probability if the response rate is 47% and there is no limiting safety issue.
I would have to look more at the data for the 5 of 28 patients.
Larry, thank you, I see your risk management background on this.
This is much more accurate analysis towards pointing to efficacy. I wish you were heard more.
I spent some time calculating the likely slope of the 10% of the population that has the 10Yr progression and the daily decline is close to -0.0065 very close from the -0.007 for responders.
Essentially the trial got 47% that during 9 months showed a progression similar to the 10 yr ALS patients.
I also estimated the probability of getting such 7 out of 15 that match the 10 yr profile and I got more than 4 STDs from the expected average (I used a little inflated around 2.09). Thus it looks like the probability of picking 7 out of 15 at random is very low.
Even if there are some things on the eligibility criteria that would make the expected higher than 10% like 20% it is still far away from average.
Even with being 9 months this would at least give the patients 5 months considering 100% of the patients then goes to normal progression which I think it is unlikely.
Some analysts are looking at averages and speed of decline and STD of the average putting responders and non responders together.
Thanks a lot for this!
Thanks for the insight. It seems to me that there is almost no questions that we are seeing a dramatic effect in what is probably a meaningful part of the ALS patient universe.
If the outcome for the non-responder group characterizes what happens to the other patients, it is troubling. However, given the non-action of the FDA and the endorsement of the data by key investigators, I suspect we will feel somewhat better about the non-responders after Feldman presents.
I think that some of the responders will go significantly beyond 9 months as in the phase 1 trial.
The Brainstorm Cell data is very flaky. The data in the period following the one time treatment has a downward slope comparable to placebo.