Gene Therapy Will Be A Major Driver of Biopharma Growth in Coming Decades
Overview
This is the fourth in a series of articles intended to give a layman’s view of the technologies that are the focus of biopharma research and will be driving commercial sales in coming decades. The explosion of scientific discoveries is breathtaking and is growing exponentially. It is far beyond my capability and that of most investors to grasp all that is going on. However, it is important to have a general understanding of these technologies. For those who despair at the problems that the world is facing with the pandemic and unrest in the US, I would offer that our ability to innovate is virtually without limit and the best is yet to come.
In the previous article on RNA interference, I hypothesized that this would offer major advances over the small molecule and monoclonal antibodies technologies that drive current research and development for the worldwide biopharma industry. The timelines for a new technology to be broadly developed are very long in biopharma owing to the lengthy time to take a drug from test tube to clinical trials, long clinical trials needed to gain regulatory approval and then to be incorporated into medical practice. For example, the first commercially important monoclonal antibody, Rituxan, was introduced in 1997. Now, 30 years later monoclonal antibody drugs are the major driver of biopharma sales and research.
The first significant RNA interference products, Alnylam’s Onpattro and Ionis/ Biogen’s Spinraza were introduced in August 2018 and December 2016. The drug development effort in RNA interference is frenetic and the pipeline of novel drugs in development is staggering. I believe there is the potential for an enormous number of blockbuster drugs to be developed in the coming years and decades. This article focuses on gene therapy which I believe has comparable potential to RNA interference. The first commercially important gene therapy products were just introduced in the last two years. These were the CAR-T products-Novartis’ Kymriah and Gilead’s Yescarta- and Novartis’s Zolgensma. As with RNA interference, I think that there is the potential for an enormous number of blockbuster drugs to be developed in the coming years and decades. RNA interference and gene therapy are on the same timeline.
My intention in writing these articles is to provide a layman’s overview of the biology underlying RNA interference and gene therapy in order to understand the many companies working in these areas. After I complete these overview reports, I will try to identify companies that have exciting investment outlooks. I think that it is likely that there will be many such opportunities driven by a successful drug development effort. I think that acquisitions of small companies by large biopharma will be the most frequent opportunity. Already, four of the leading gene therapy emerging growth companies-Kite, Spark, AveXis and Juno- were acquired before their lead products were introduced. There may be 20, 30, 50 or more companies with home run investment potential. I hope to identify a few.
The Function of Genes and Their Role in Diseases
The human body is a biological supercomputer and its operating software is DNA that is made up of nucleotide structural units. Genes are specific sequences of nucleotides located on extremely long strands of DNA called chromosomes. Humans have 46 chromosomes with 23 inherited from the mother and 23 from the father that are matched. This means that humans have two copies of each gene with one exception-the sex chromosomes are not exactly matched as a woman has XX chromosomes and the male has XY. Humans have somewhere between 20,000 and 25,000 genes in which the linear sequence of nucleotides carries instructions for the body to synthesize proteins and RNA. The genetic code written in the DNA of genes is transcribed into messenger RNA which is then used as a template to synthesize proteins in ribosomes.
The transmission of genes to an offspring is the basis of the inheritance of phenotypic traits such as hair color, height and so on. The DNA sequences of which a gene is formed is called the genotype and along with environmental and developmental factors determines the phenotype. Most biological traits are determined by a complex interaction of a number of different genes. Some phenotypes are visible, such as eye color or the number of limbs, and some are not, such as blood type, risk for specific diseases, or the thousands of basic biochemical processes that constitute life.
Sometimes genes are flawed and many diseases are driven by mutations in which the mutated genes can result in the production of disease causing proteins. It is often the case that mutated genes are inherited from one or both parents that result in genetic disease such as cystic fibrosis and multiple sclerosis. However, mutations can also randomly occur during cell division or owing to external toxins such as chemicals, ultraviolet light and viral and bacterial infections. A small alteration in the DNA nucleotide base sequence of a gene can significantly alter the structure of the protein it codes for so that it does not function properly and causes a disease.
The Promise of Gene Therapy
Genes are the blueprints for protein production and if the blueprint is flawed, disease can result. Gene therapy is the purposeful introduction, removal or alteration of genetic material in the gene to change or possibly eliminate the protein produced. Gene therapy may be able to reduce production of disease causing proteins or increase production of disease fighting proteins or even produce new or modified proteins.
A major promise of gene therapy is that instead of providing proteins or other therapies externally with pills or injections that need to be dosed over a lifetime, gene therapy offers the possibility of dosing a patient once to achieve a long‑term, durable benefit. Once the therapeutic gene is transferred to a patient’s cells, the cells may be able to continue to produce the therapeutic protein for years or, potentially, the rest of the patient’s life. As a result, gene therapy has the potential to transform the way these patients are treated by addressing the underlying genetic defect.
The most common current way that genetic material is transferred to the nucleus of cells is using a virus as a carrier or vector to deliver the gene. Viruses are used because their very existence is based on penetrating a cell and getting the genome of that cell to incorporate its genetic material and express proteins that it needs to survive and spread. Through genetic engineering, the infection causing part of the virus is removed so that when it enters the nucleus of the cell to deliver its genetic payload, it does not make the patient sick.
There are two kinds of gene therapy-in vivo (in the body) and ex vivo (outside the body). With in vivo therapy, the drug is injected directly into the bloodstream or other tissue. In ex vivo therapy, the genes are added to the cells in a laboratory, the cells are expanded exponentially and then returned to the patient. The approach chosen depends on the disease and the tissue in which it occurs.
The new technologies of CRISPR and zinc finger nucleases hold significant promise to improve gene therapy because of their ability to precisely insert and alter genes. While both are effective techniques, CRISPR lends itself to much easier development. Many consider CRISPR to be one of the most important discoveries in the history of biology.
The Era of Gene Therapy Has Finally Begun
After extensive research on animals throughout the 1980s, the first approved gene therapy clinical trial in the US started in 1990. This was the first direct insertion of human DNA into the genome to treat a patient with ADA-SCID (bubble boy disease). It is estimated that since then over 2,900 clinical trials have been conducted, with more than half of those being phase 1 trials. The whole field suffered a major setback in 1991 when Jesse Geisinger was treated for a non-life threatening genetic disease and died from an immune response to the treatment. As a result, the FDA suspended several clinical trials pending the reevaluation of ethical and procedural practices and research was stalled for over a decade.
The first commercial gene therapy, Gendicine, was approved in China in 2003 for the treatment of certain cancers. In 2011, Neovasculgen was registered in Russia for the treatment of peripheral artery disease, including critical limb ischemia. In 2012, UniQure’s Glybera was the first product approved in Europe for the treatement of the rare inherited disorder, lipoprotein lipase deficiency. None of these products were commercially successful and none were approved by the FDA.
The FDA recently has approved four gene therapy products:
- Novartis’s Kymriah in August 2017
- Gilead’s Yescarta (obtained through the acquisition of Kite) in October 2017
- Roche’s Luxturna (via the Spark Therapeutics acquisition) May 2019 and
- Novartis’s Zolgensma (through the AveXis acquisition) in March 2020.
- In addition. I expect the FDA will soon approve two products of Bristol-Myers Squibb. These are the CAR-T products liso pro and bb 2121 that were developed by Juno which was acquired by Celgene that in turn was acquired by BMY.
In later reports, I will go into more thinking on how to invest in the numerous companies involved in gene therapy. Based on the experience thus far, it is evident that bigger biopharma companies are extremely interested and are willing to make huge investments to gain access to the technologies as shown below:
- Gilead acquired Kite in August of 2017 for $11.9 billion; this was prior to the approval of Yescarta in October of 2017.
- Roche paid $4.8 billion for Spark in February 2020 even though Luxturna has achieved minimal sales.
- Novartis paid $8.7 billion in April 2019 for AveXis prior to the approval of Zolgensma
- Celgene paid $9.0 billion for Juno in January of 2018 and products obtained in that acquisition are just now pending approval.
Background on Early Gene Therapy Products
Luxturna (voretigene neparvovec-rzyl) delivers a gene to the retina that expresses the protein RPE 65. Inadequate production of RPE 65 causes the rare genetic disease, Leber's congenital amaurosis, and results in impaired vision or blindness. Zolgensma (onasemnogene abeparvovec)introduces a gene which treats spinal muscular atrophy, a life threatening disease in which control over muscles is impaired. It results from inadequate production of the SMN protein due to malfunctioning of the SMN1 gene. Zolgensma is designed to deliver a fully functional human SMN1 gene into the nuclei of target cells, including motor neurons to increase SMN protein levels. Results have been spectacular from both a medical and commercial standpoint for Zolgensma. Luxturna’s commercial results have been limited.
Both Luxturna and Zolgensma are in vivo gene therapy products given by infusion into the body. There have been two ex vivo products that have been approved. These are the CAR-T products- Gilead’s Yescarta (axicabtagene ciloleucel) and Novartis’s Kymriah (tisagenlecleucel). These are genetically engineered T-cells. T-cells are key cells of the adaptive immune system. Their evolutionary function is to fight off infections, but they also can detect and kill cancer cells. However, cancers can develop ways to weaken the effects of T-cells- CAR-T technology can restore efficacy.
T-cells are taken from the patient and are then genetically engineered in a laboratory to express chimeric receptors on their surface that can recognize a cancer antigen and target the T-cell to the cancer. CAR-T technology alters T-cells by harvesting a patient’s white blood cells in a process called leukapheresis. Gene sequences are constructed and then transferred into the T cell’s DNA using a viral vector. The number of CAR-T cells is then expanded until they reach the desired dose level and infused back into the patient
There are numerous other products in various stages of development and close to commercialization. One of the most anticipated is BioMarin’s Valrox (valoctocogene roxaparvovec) which is an in vivo treatment for hemophilia A which is caused by lack of production of clotting factor VIII. An introduction is expected by yearend 2020. In the CAR-T space there is feverish competition by a broad range of companies to develop better CAR-T drugs. Bristol-Myers Squibb should receive approval for liso-pro (a direct competitor to Yescarta and Kymriah) late in 2020 and in 2021 approval for bb 2121 is expected. These are a vast number of other gene therapy products in the biopharma pipeline.
Design of Gene Therapy Products
Vectors, Transgenes and Promoters
The current and most advanced gene therapy products are comprised of a vector, a promoter, and a transgene; identifying and combining the appropriate components is critical to developing viable products. The transgene is the genetic material introduced into targeted tissues by the vector, and its expression is driven by the promoter. Transgenes vary depending on the disease and protein of interest. Some transgenes contain the full copy of the gene of interest; whereas others contain a partial gene. The role of the promoter is to drive selective gene expression in intended tissue targets.
The vector is responsible for tissue targeting and delivering the transgene and the promoter to target cells. There are several different types of vectors, including viral vectors such as adenovirus, adeno-associated virus, retrovirus or lentivirus. Other non-viral vectors used in gene therapy include bacterial, lipid- and polymer-based vectors. Patients can potentially have antibodies to some vectors and consideration of immune response is important in selecting a vector.
Luxturna, Zolgensma, Kymriah and Yescarta all use viral vectors to insert genes into the genome of a cell. This could involve removing a disease causing gene from the genome, adding a gene to produce a missing protein (like Luxturna and Zolgensma) or correct a mutation in a gene that causes disease. The most exciting of these technologies is CRISPR which I will discuss in a later section. In my opinion, this is the most exciting new technology in biotechnology. Sangamo has been working for many years on another gene editing technology called zinc finger nucleases. It is not as easy to work with as CRISPR, but is a viable technique.
Somatic Versus Germ Line Gene Therapy
All of the products I have discussed so far address diseases in somatic cells which are cells that are not passed on to offspring. However, gene therapy can also be used to add or delate genes in germ cells which are passed on to offspring. The ethical issues with this are obvious and there are hot debates on what type of restrictions should be placed on this technology. There is more than a theoretical potential for creating designer babies with genes to enhance intelligence or appearance. There has already been one such experiment in China. On the other hand, gene editing of stem cells that are not germ cells holds enormous promise such as creating new pancreases for type 1 diabetics or new kidneys or hearts. This is not science fiction. It is a very real and inevitable.
Viral Vectors Used in Gene Therapy
Gene therapy is based on the delivery of genes (DNA sequences) into the nucleus of a patient's cells. A DNA molecule is packaged within a viral vector in order to transport it into the nucleus of human cells. Viral vectors used in gene therapies are typically comprised of: a transgene (the DNA sequence) and a promoter that are packaged in a delivery mechanism called a capsid. This builds on the key biological attributes of viruses which is to introduce their genetic material into the host cell and then use the host's cellular machinery to manufacture viral proteins. A number of viruses have been used for human gene therapy, including retroviruses, adenoviruses, herpes simplex, vaccinia, and adeno-associated virus.
A virus (from the Latin virus meaning toxin or poison) is a microscopic organism consisting of genetic material (RNA or DNA) surrounded by a protein forming a capsid. This capsid in turn may be surrounded by a lipid membrane. Typically, virus particles are 100 times smaller than a single bacterial cell and a bacterial cell is more than 10 times smaller than a human cell. They reproduce by infecting a host cell and then using host cell mechanisms to replicate themselves. Interestingly, this raises the question as to whether they are actually living microorganisms.
A viral infection starts with the virus attaching to a receptor on the surface of a host cell. The virus then enters the cell and travels to the nucleus. There it may use the same enzymes that the host cell uses in DNA replication to transcribe the viral DNA and make new viral particles. Other cellular mechanisms of the host cell are then used to assemble the particles to make new viruses. The newly formed viruses may then spread through shedding from the host cell or killing the host cell and releasing its viral contents. Gene delivery takes advantage of certain viral properties to introduce genes into human host cells.
In gene therapy a gene that is intended for delivery is packaged into a replication-deficient viral particle to form a viral vector. Virus mediated gene delivery contains the desired gene and removes the part of the virus’ genome that is infectious. Virus-mediated insertion of DNA into the host cell is called transduction. Successful gene delivery requires the genetic material to remain stable within the host cell and replicate when the cell divides. Some viruses integrate into the DNA and become part of the genome like the lentiviruses used in CAR-T therapy. Others like adeno associated virus used with Zolgensma and Luxturna do not integrate into the genome but do replicate with the cell.
Gene transfer using adeno associated viruses (AAVs) can target specific cells to correct improper protein functioning that causes disease. Once inside the cell the carrier can provide copies of a gene that corrects the missing protein function. To do this AAV generic material is replaced with a gene that restores normal function of the disrupted protein. A protein shell called the capsid encloses the genetic material and helps target delivery to certain cells. Once inside, the capsule is shed inside the nucleus and releases its genetic material. This genetic material does not integrate into the DNA of the cell. Instead, it forms episomes. Using the body’s natural machinery, this allows stable long term production of the therapeutic molecule. AAV systems can be administered by intravenous drip or by direct injection into targeted tissues. AAVs are ideal carriers for gene transfer as they do not cause disease and are capable of targeting both dividing and non-dividing cells.
Retroviruses are RNA viruses that carry a gene for a reverse transcriptase that transcribes the viral genetic material into a double stranded DNA intermediate. This DNA intermediate is then incorporated into the host DNA allowing the host cell machinery to produce all the necessary viral components. Additionally, because the viral genome is stably integrated into the host DNA, any modification that has been made will be passed to all daughter cells that are derived from the transfected cell currently; the most common retrovirus used is derived from the murine leukemia virus. In general, retroviruses have been used for ex vivo gene therapy applications as they are unable to efficiently infect non-dividing cells.
Issues for Gene Therapy
Gene therapy faces significant challenges, some of which are as follows:
- Short-lived nature: Before gene therapy can become a permanent cure for a condition, the therapeutic DNA introduced into target cells must remain functional and the cells containing the therapeutic DNA must be stable. Problems with integrating therapeutic DNA into the genome and the rapidly dividing nature of many cells may prevent achieving long-term benefits so requiring patients to receive multiple treatments.
- Immune response: Any time a foreign object like a viral vector is introduced into human tissues, the immune system may be stimulated to attack the invader. Stimulating the immune system in a way that reduces gene therapy effectiveness is possible. The immune system's enhanced response to viruses which it has seen before reduces the effectiveness to repeated treatments.
- Side effect issues: Some viral vectors carry the risks of toxicity and cause immune responses.
- Multigene disorders are more complicated to address: Current therapies are focused on monogenic diseases which are caused by mutation of a single gene. Many commonly occurring disorders, such as heart disease, high blood pressure, Alzheimer's disease, arthritis, and diabetes, are affected by variations in multiple genes, which greatly complicate gene therapy.
- Altering germ line cells: Some therapies may breach the barrier between somatic cells and affect germ the germline. Modifications of germ line cells results in the genetic alteration being passed on to future generations, an area of both medical and ethical concern,
- Insertional mutagenesis: Some gene therapy approaches integrate the gene randomly into a patient’s genome. If the DNA is integrated in a sensitive spot in the genome, for example in or near a tumor suppressor gene, the therapy could induce a tumor. This has occurred in clinical trials for X-linked severe combined immunodeficiency patients, in which hematopoietic stem cells were transduced with a corrective transgene using a retrovirus, and this led to the development of T cell leukemia in 3 of 20 patients.
- Cost: Zolgensma is priced at $2.1 million per year and there are estimates that Valrox will be priced at $3 million for a single dose. This requires a whole new approach for reimbursement versus conventional drugs.
Gene Editing Using CRISPR
First discovered in 2012, the CRISPR Cas9 system for gene editing is widely regarded as the most promising technique for gene editing because it is fast, cheap, precise and relatively easy to use. The CRISPR (acronym for clustered regularly-interspaced short palindromic repeats) system was first identified in bacteria as a way of defending against viral infections. It is an immune response although extremely primitive in comparison to the human immune system. Bacteria use CRISPR to chop up and destroy the DNA of viruses. Researchers have developed ways to use this system to disrupt, delete and alter genes to achieve a therapeutic effect or to insert an entirely new gene.
The process starts with the synthesis of an RNA sequence (guide strand) whose nucleotides are complementary to a segment of DNA in the human genome. The guide strand is attached to an enzyme called Cas9 (CRISPR-associated protein 9). After the guide strand attaches to the complementary DNA strand in the human genome, Cas9 will cut the DNA at both ends thus removing it from the genome. Once the DNA is cut, the cell's natural repair mechanisms activate to repair the break. One repair method involves gluing the two cuts back together; this method is known as non-homologous end joining. It tends to introduce errors as nucleotides may be accidentally inserted or deleted, resulting in mutations, which could disrupt a gene. A second method fixes the break by filling in the gap with a sequence of nucleotides using a short strand of DNA as a template. Scientists can supply the DNA template of their choosing, thereby writing-in any gene they want, or correcting a mutation. In this way scientists can alter or stop the functioning of a gene or insert an entirely new gene.
CRISPR Therapeutics was the first company to use the CRISPR technology to actually treat a human. In July 2019, a patient was treated for sickle cell disease. The patient was enthusiastic about the results of the ex vivo treatment and is now being monitored to determine safety issues, if any, and duration of effect. CRISPR is now conducting phase 1/2 trials in β thalassemia and sickle cell disease with 45 patients enrolled in each disease. Editas first treated a patient suffering from the blindness causing, genetic disease Leber’s congenital amaurosis in March 2020 and is conducting a phase 1 trial in 18 patients.
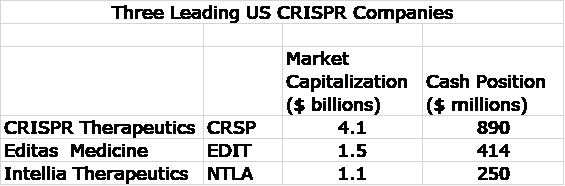
There are three major US companies leading the development effort in CRSPR systems. They are carrying significant market capitalizations and are well capitalized. Obviously, the investment community sees great potential. I would not be surprised to see each of these companies acquired by big pharma at very significant premiums to current valuations in the next few years.
Tagged as Gene therapy + Categorized as LinkedIn, Smith On Stocks Blog





How about ADVM and RGNX?