Cytokinetics: Tirasemtiv May Still Be Successfully Developed in ALS (CYTK, Buy, $4.77)
I think that there is a strong possibility that Cytokinetics (to the surprise of Wall Street) could announce sometime in coming months that it will undertake a phase 3 registrational trial for tirasemtiv in ALS. After tirasemtiv failed to reach the primary endpoint of ALSFRS-r in the phase 2 BENEFIT-ALS trial, the stock crashed and investors wrote off tirasemtiv. I laid out my detailed thinking on why I think a phase 3 trial might be in the cards in my recent report Cytokinetics: I Think That the Company May Decide to Do A Phase 3 Trial with Tirasemtiv. I would urge you to review that report. Earlier this week, I listened to a presentation by CEO Robert Blum and then spoke with him afterwards. This reinforced my view.
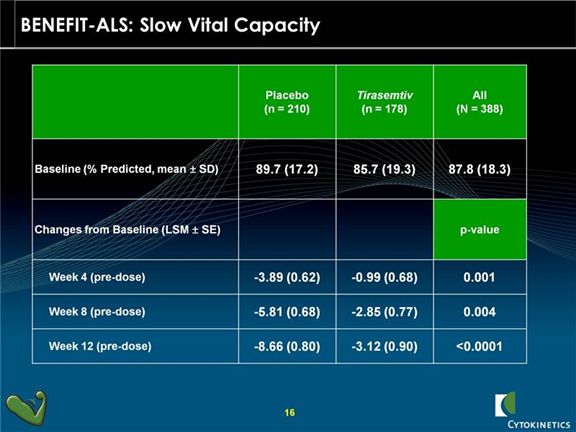
In his presentation, Mr. Blum characterized the BENEFIT-ALS trial as the most successful trial in ALS in the last 20 years and emphasized the importance of slow vital capacity (SVC) in assessing the benefit of tirasemtiv. He stated that many key opinion leaders believe that SVC should be accepted by regulators as the primary endpoint of a new phase 3 trial. They point out that this is the most important indicator of respiratory function and respiratory failure is almost always the cause of death in ALS. SVC was a prospectively defined secondary endpoint in the BENEFIT-ALS trial and the results were outstanding as tirasemtiv showed very exceptionally strong p-values at 4, 8 and 12 weeks (see the chart below). Based on this, I would think that the chances of reaching statistical significance with SVC as an end point in a phase 3 trial are excellent and I think that with the support of data from BENEFIT-ALS that one successful phase 3 trial would be enough to file for regulatory approval.
Slow vital capacity is a measure of the maximum amount of air that a person is capable of slowly expelling from their lungs after taking the deepest breath that they can. Normal adults can exhale between three and five liters of air. SVC is not a measure of how fast, but how completely this slow exhalation can be done. Patients breathe into a commonly used device for measuring pulmonary function called a spirometer. This measures the amount of air exhaled and then uses algorithms based on age, gender, height, weight and ethnicity to measure slow vital capacity relative to what a normal person might do. It reads out a percentage of the level of SVC for a patient as compared to the expected level for a normal person of similar characteristics.
SVC is an indicator of the strength of the skeletal muscles that are responsible for breathing. It is linked to measures of disease progression and is a predictor of survival in ALS patients. It is the respiratory measure that is most commonly associated with death and is used by friends and caregivers to plan for the death of patients. ALS patients know that more than any other thing this measures their disease progression. In the same way that most people know their social security numbers, ALS patients know their slow vital capacity scores; it is measured at every visit. It is also a determinant factor in in the decision to include patients in clinical trials and to determine when there is a need for ventilatory assistance.
Slow vital capacity was one of the pre-specified secondary endpoints in BENEFIT-ALS and showed a very strong p<0.0006 at the end of this 12 week trial. There was less of a decline in vital capacity for tirasemtiv patients at assessment time points of 4, 8 and 12 weeks indicating a sustained and durable benefit. On an overall basis, the rate of decline was one-third that of control patients.
Mr. Blum seemed to indicate, although he gave no precise guidance, that meetings with regulatory agencies will take place in coming months to see if the Company can gain their concurrence that SVC is an acceptable primary endpoint. In the US, the Company will almost certainly seek a Special Protocol Assessment (SPA).
If the FDA agrees that SVC is an acceptable primary endpoint , I can see a dramatic move in the stock. The exceptional results obtained on SVC in BENEFIT-ALS would give strong encouragement to investors that there is an excellent chance of being successful in a phase 3 trial in which SVC is the primary endpoint. I could see the stock price increasing to $6.00 to $8.00 on this news. I think we could hear on whether this is a possibility in the next three to six months.
If the FDA does not agree that SVC is an acceptable primary end point and insists on ALSFRS-r, I think that tirasemtiv development will be abandoned. I don’t think there is much, if any; expectation for tirasemtiv in the current stock price so dropping development might not have a noticeable impact on the stock. Dropping tirasemtiv would not, however, be the end of Cytokinetics’ effort to develop a drug for ALS. CK-107 is the third drug in human trials for CYTK and could begin phase 2 trials later this year. Its mode of action is comparable to tirasemtiv and it could potentially be developed in ALS. CK-107 is being developed in a partnership with Astellas.
Tagged as BENEFIT-ALS, cytk, Cytokientics, tirasemtiv + Categorized as Smith On Stocks Blog





If FDA only requires SVC, then it will be a complete victory. But I am afraid FDA may want to see OS benefits instead of a surrogate indicator. OS will always be a viable primary endpoint of life-threatening diseases but it will drag on the trial one year or two and no one can be sure that a surrogate indicator really correlates with survival, adding some uncertainty to the fate of tirasemtiv if that scenario happens.
Anyway, it can’t be worse given current stock price this low. And I do hope FDA soft on this one, which may benefit NSI 566 trial III as well.
I think that with most diseases, the FDA would not accept a surrogate measure like SVC as an end point. They would want to see a valid disease point like overall survival or prolonging the time to mechanical ventilation. The most dangerous words in the English language are “This time it is different.” However, this is ALS and there has been no new drug approved for 20 years and that drug, rilutek, is marginally effective. The FDA is under great pressure from the ALS community to be aggressive in considering new drugs. Also, SVC appears to be a very good surrogate marker. If an ALS patient’s breathing function declines at a lesser rate even for only a few months as compared to placebo, most people would accept this as a sign of efficacy.
I don’t know what the FDA will do, but some opinion leaders are urging this course of action and there will be howls of protest from the ALS community if the FDA sticks to ALSFRS-r as a primary endpoint. However’ as you so correctly point out, acceptance of SVC as the primary endpoint would be a complete victory. If the FDA insists on using ALSFRS-r as the endpoint tirasemtiv is dead, but again as you point out, there is not much in the stock for tirasemtiv.
Thanks as always for your perceptive comments.